Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia
- PMID: 39408979
- PMCID: PMC11476590
- DOI: 10.3390/ijms251910651
Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia
Abstract
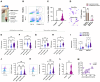
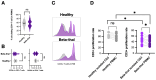
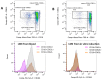
Neutrophil dysfunction is a form of immune suppression in patients with β-thalassemia (Beta-thal), although data on this are limited. In this study, blood from patients and healthy volunteers was analyzed. Flow cytometry analysis demonstrated an increase in immature neutrophils (CD16- CD62L+) and aged (senescent) neutrophils (CD16+ CD62L-) in Beta-thal patients compared to healthy volunteers. The Beta-thal neutrophils demonstrated less prominent chemotaxis and phagocytosis than healthy neutrophils at the baseline. With phorbol myristate acetate (PMA) or lipopolysaccharide (LPS) stimulations, some of the indicators, including the flow cytometry markers (CD11b, CD62L, CD66b, CD63, apoptosis, and reactive oxygen species) and neutrophil extracellular traps (NETs; detected by anti-citrullinated histone 3 immunofluorescence), were lower than the control. Additionally, low-density neutrophils (LDNs), which are found in the peripheral blood mononuclear cell (PBMC) fraction, were observed in Beta-thal patients but not in the control group. The expression of CD11b, CD66b, CD63, arginase I, and ROS in LDNs was higher than the regular normal-density neutrophils (NDNs). The proliferation rate of CD3+ T cells isolated from the PBMC fraction of healthy volunteers was higher than that of the cells from patients with Beta-thal. The incubation of red blood cell (RBC) lysate plus ferric ions with healthy NDNs transformed the NDNs into the aged neutrophils (decreased CD62L) and LDNs. In conclusion, iron overload induces neutrophil diversity along with some dysfunctions.
Keywords: iron overload; low-density neutrophils; neutrophil diversity; thalassemia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Sae-Khow K., Charoensappakit A., Visitchanakun P., Saisorn W., Svasti S., Fucharoen S., Leelahavanichkul A. Pathogen-Associated Molecules from Gut Translocation Enhance Severity of Cecal Ligation and Puncture Sepsis in Iron-Overload β-Thalassemia Mice. J. Inflamm. Res. 2020;13:719–735. doi: 10.2147/JIR.S273329. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- the 90th Anniversary of Chulalongkorn University/Chulalongkorn University
- N41A640076/the National Research Council of Thailand (NRCT)
- N34A660583/the National Research Council of Thailand (NRCT)
- B16F640175/the Program Management Unit for Human Resources, Institutional Development, Research, and Innovation
- HEA663000017/the Thailand Science research and Innovation Fund, Chulalongkorn University
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

