Trehalose Attenuates In Vitro Neurotoxicity of 6-Hydroxydopamine by Reducing Oxidative Stress and Activation of MAPK/AMPK Signaling Pathways
- PMID: 39408988
- PMCID: PMC11476739
- DOI: 10.3390/ijms251910659
Trehalose Attenuates In Vitro Neurotoxicity of 6-Hydroxydopamine by Reducing Oxidative Stress and Activation of MAPK/AMPK Signaling Pathways
Abstract
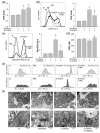
The effects of trehalose, an autophagy-inducing disaccharide with neuroprotective properties, on the neurotoxicity of parkinsonian mimetics 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenylpiridinium (MPP+) are poorly understood. In our study, trehalose suppressed 6-OHDA-induced caspase-3/PARP1 cleavage (detected by immunoblotting), apoptotic DNA fragmentation/phosphatidylserine externalization, oxidative stress, mitochondrial depolarization (flow cytometry), and mitochondrial damage (electron microscopy) in SH-SY5Y neuroblastoma cells. The protection was not mediated by autophagy, autophagic receptor p62, or antioxidant enzymes superoxide dismutase and catalase. Trehalose suppressed 6-OHDA-induced activation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and AMP-activated protein kinase (AMPK), as revealed by immunoblotting. Pharmacological/genetic inhibition of JNK, p38 MAPK, or AMPK mimicked the trehalose-mediated cytoprotection. Trehalose did not affect the extracellular signal-regulated kinase (ERK) and mechanistic target of rapamycin complex 1 (mTORC1)/4EBP1 pathways, while it reduced the prosurvival mTORC2/AKT signaling. Finally, trehalose enhanced oxidative stress, mitochondrial damage, and apoptosis without decreasing JNK, p38 MAPK, AMPK, or AKT activation in SH-SY5Y cells exposed to MPP+. In conclusion, trehalose protects SH-SY5Y cells from 6-OHDA-induced oxidative stress, mitochondrial damage, and apoptosis through autophagy/p62-independent inhibition of JNK, p38 MAPK, and AMPK. The opposite effects of trehalose on the neurotoxicity of 6-OHDA and MPP+ suggest caution in its potential development as a neuroprotective agent.
Keywords: 6-hydroxydopamine; AMP-activated protein kinase; JNK; MPP+; Parkinson’s disease; mitochondrial damage; oxidative stress; p38 MAPK; trehalose.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Muleiro Alvarez M., Cano-Herrera G., Osorio Martínez M.F., Vega Gonzales-Portillo J., Monroy G.R., Murguiondo Pérez R., Torres-Ríos J.A., van Tienhoven X.A., Garibaldi Bernot E.M., Esparza Salazar F., et al. A comprehensive approach to Parkinson’s disease: Addressing its molecular, clinical, and therapeutic aspects. Int. J. Mol. Sci. 2024;25:7183. doi: 10.3390/ijms25137183. - DOI - PMC - PubMed
-
- Blum D., Torch S., Lambeng N., Nissou M., Benabid A.L., Sadoul R., Verna J.M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001;65:135–172. doi: 10.1016/S0301-0082(01)00003-X. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 451-03-47/2023-01/200110/Ministry of Science, Technological Development and Innovation, Republic of Serbia
- 451-03-66/2024-03/200110/Ministry of Science, Technological Development and Innovation, Republic of Serbia
- 451-03-66/2024-03/200007/Ministry of Science, Technological Development and Innovation, Republic of Serbia
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

