Epigenetic Reprogramming and Inheritance of the Cellular Differentiation Status Following Transient Expression of a Nonfunctional Dominant-Negative Retinoblastoma Mutant in Murine Mesenchymal Stem Cells
- PMID: 39409007
- PMCID: PMC11476944
- DOI: 10.3390/ijms251910678
Epigenetic Reprogramming and Inheritance of the Cellular Differentiation Status Following Transient Expression of a Nonfunctional Dominant-Negative Retinoblastoma Mutant in Murine Mesenchymal Stem Cells
Abstract
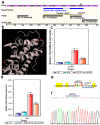
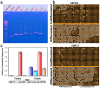
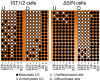
The retinoblastoma gene product (Rb1), a master regulator of the cell cycle, plays a prominent role in cell differentiation. Previously, by analyzing the differentiation of cells transiently overexpressing the ΔS/N DN Rb1 mutant, we demonstrated that these cells fail to differentiate into mature adipocytes and that they constitutively silence Pparγ2 through CpG methylation. Here, we demonstrate that the consequences of the transient expression of ΔS/N DN Rb1 are accompanied by the retention of Cebpa promoter methylation near the TSS under adipogenic differentiation, thereby preventing its expression. The CGIs of the promoters of the Rb1, Ezh2, Mll4, Utx, and Tet2 genes, which are essential for adipogenic differentiation, have an unmethylated status regardless of the cell differentiation state. Moreover, Dnmt3a, a de novo DNA methyltransferase, is overexpressed in undifferentiated ΔS/N cells compared with wild-type cells and, in addition to Dnmt1, Dnmt3a is significantly upregulated by adipogenic stimuli in both wild-type and ΔS/N cells. Notably, the chromatin modifier Ezh2, which is also involved in epigenetic reprogramming, is highly induced in ΔS/N cells. Overall, we demonstrate that two major genes, Pparγ2 and Cebpa, which are responsible for terminal adipocyte differentiation, are selectively epigenetically reprogrammed to constitutively silent states. We hypothesize that the activation of Dnmt3a, Rb1, and Ezh2 observed in ΔS/N cells may be a consequence of a stress response caused by the accumulation and malfunctioning of Rb1-interacting complexes for the epigenetic reprogramming of Pparγ2/Cebpa and prevention of adipogenesis in an inappropriate cellular context. The failure of ΔS/N cells to differentiate and express Pparγ2 and Cebpa in culture following the expression of the DN Rb1 mutant may indicate the creation of epigenetic memory for new reprogrammed epigenetic states of genes.
Keywords: Cebpa; DNMTs; MSCs; Rb1; adipogenesis; epigenetic reprogramming.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

