SILAC-Based Characterization of Plasma-Derived Extracellular Vesicles in Patients Undergoing Partial Hepatectomy
- PMID: 39409015
- PMCID: PMC11476990
- DOI: 10.3390/ijms251910685
SILAC-Based Characterization of Plasma-Derived Extracellular Vesicles in Patients Undergoing Partial Hepatectomy
Abstract
Post-hepatectomy liver failure (PHLF) remains a significant risk for patients undergoing partial hepatectomy (PHx). Reliable prognostic markers and treatments to enhance liver regeneration are lacking. Plasma nanoparticles, including lipoproteins, exosomes, and extracellular vesicles (EVs), can reflect systemic and tissue-wide proteostasis and stress, potentially aiding liver regeneration. However, their role in PHLF is still unknown.
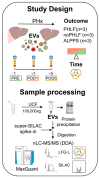
Methods: Our study included nine patients with hepatocellular carcinoma (HCC) undergoing PHx: three patients with PHLF, three patients undergoing the associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure, and three matched controls without complications after PHx. Patient plasma was collected before PHx as well as 1 and 5 days after. EVs were isolated by ultracentrifugation, and extracted proteins were subjected to quantitative mass spectrometry using a super-SILAC mix prepared from primary and cancer cell lines.
Results: We identified 2625 and quantified 2570 proteins in the EVs of PHx patients. Among these, 53 proteins were significantly upregulated and 32 were downregulated in patients with PHLF compared to those without PHLF. Furthermore, 110 proteins were upregulated and 78 were downregulated in PHLF patients compared to those undergoing ALPPS. The EV proteomic signature in PHLF indicates significant disruptions in protein translation, proteostasis, and intracellular vesicle biogenesis, as well as alterations in proteins involved in extracellular matrix (ECM) remodelling and the metabolic and cell cycle pathways, already present before PHx.
Conclusions: Longitudinal proteomic analysis of the EVs circulating in the plasma of human patients undergoing PHx uncovers proteomic signatures associated with PHLF, which reflect dying hepatocytes and endothelial cells and were already present before PHx.
Keywords: extracellular vesicles; liver regeneration; post-hepatectomy liver failure; proteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Santol J., Ammann M., Reese T., Kern A.E., Laferl V., Oldhafer F., Dong Y., Rumpf B., Vali M., Wiemann B., et al. Comparison of the LiMAx Test vs. the APRI + ALBI Score for Clinical Utility in Preoperative Risk Assessment in Patients Undergoing Liver Surgery—A European Multicenter Study. Eur. J. Surg. Oncol. 2024;50:108048. doi: 10.1016/j.ejso.2024.108048. - DOI - PubMed
-
- Schwarz C., Plass I., Fitschek F., Punzengruber A., Mittlböck M., Kampf S., Asenbaum U., Starlinger P., Stremitzer S., Bodingbauer M., et al. The Value of Indocyanine Green Clearance Assessment to Predict Postoperative Liver Dysfunction in Patients Undergoing Liver Resection. Sci. Rep. 2019;9:8421. doi: 10.1038/s41598-019-44815-x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

