Biocompatible Poly(ε-Caprolactone) Nanocapsules Enhance the Bioavailability, Antibacterial, and Immunomodulatory Activities of Curcumin
- PMID: 39409022
- PMCID: PMC11476408
- DOI: 10.3390/ijms251910692
Biocompatible Poly(ε-Caprolactone) Nanocapsules Enhance the Bioavailability, Antibacterial, and Immunomodulatory Activities of Curcumin
Abstract
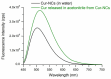
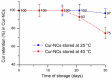
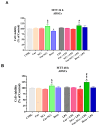
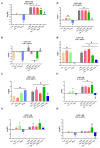
Curcumin (Cur), the primary curcuminoid found in Curcuma longa L., has garnered significant attention for its potential anti-inflammatory and antibacterial properties. However, its hydrophobic nature significantly limits its bioavailability. Additionally, adipose-derived stem cells (ADSCs) possess immunomodulatory properties, making them useful for treating inflammatory and autoimmune conditions. This study aims to verify the efficacy of poly(ε-caprolactone) nanocapsules (NCs) in improving Cur's bioavailability, antibacterial, and immunomodulatory activities. The Cur-loaded nanocapsules (Cur-NCs) were characterized for their physicochemical properties (particle size, polydispersity index, Zeta potential, and encapsulation efficiency) and stability over time. A digestion test simulated the behavior of Cur-NCs in the gastrointestinal tract. Micellar phase analyses evaluated the Cur-NCs' bioaccessibility. The antibacterial activity of free Cur, NCs, and Cur-NCs against various Gram-positive and Gram-negative strains was determined using the microdilution method. ADSC viability, treated with Cur-NCs and Cur-NCs in the presence or absence of lipopolysaccharide, was analyzed using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide assay. Additionally, ADSC survival was assessed through the Muse apoptotic assay. The expression of both pro-inflammatory (interleukin-1β and tumor necrosis factor-α) and anti-inflammatory (IL-10 and transforming growth factor-β) cytokines on ADSCs was evaluated by real-time polymerase chain reaction. The results demonstrated high stability post-gastric digestion of Cur-NCs and elevated bioaccessibility of Cur post-intestinal digestion. Moreover, Cur-NCs exhibited antibacterial activity against Escherichia coli without affecting Lactobacillus growth. No significant changes in the viability and survival of ADSCs were observed under the experimental conditions. Finally, Cur-NCs modulated the expression of both pro- and anti-inflammatory cytokines in ADSCs exposed to inflammatory stimuli. Collectively, these findings highlight the potential of Cur-NCs to enhance Cur's bioavailability and therapeutic efficacy, particularly in cell-based treatments for inflammatory diseases and intestinal dysbiosis.
Keywords: ADSCs; LPS; antibacterial activity; bioaccessibility; curcumin; cytokines; immunomodulatory activity; inflammation; poly(ε-caprolactone) nanocapsules; probiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

