MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells
- PMID: 39409031
- PMCID: PMC11476635
- DOI: 10.3390/ijms251910702
MiR-146a Is Mutually Regulated by High Glucose-Induced Oxidative Stress in Human Periodontal Ligament Cells
Abstract
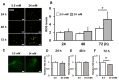
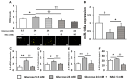
The high-glucose conditions caused by diabetes mellitus (DM) exert several effects on cells, including inflammation. miR-146a, a kind of miRNA, is involved in inflammation and may be regulated mutually with reactive oxygen species (ROS), which are produced under high-glucose conditions. In the present study, we used human periodontal ligament cells (hPDLCs) to determine the effects of the high-glucose conditions of miR-146a and their involvement in the regulation of oxidative stress and inflammatory cytokines using Western blotting, PCR, ELISA and other methods. When hPDLCs were subjected to high glucose (24 mM), cell proliferation was not affected; inflammatory cytokine expression, ROS induction, interleukin-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) expression increased, but miR-146a expression decreased. Inhibition of ROS induction with the antioxidant N-acetyl-L-cysteine restored miR-146a expression and decreased inflammatory cytokine expression compared to those under high-glucose conditions. In addition, overexpression of miR-146a significantly suppressed the expression of the inflammatory cytokines IRAK1 and TRAF6, regardless of the glucose condition. Our findings suggest that oxidative stress and miR-146a expression are mutually regulated in hPDLCs under high-glucose conditions.
Keywords: ROS; hPDLCs; high glucose; miR-146a.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

