Pentadecanoic Acid-Releasing PDMS: Towards a New Material to Prevent S. epidermidis Biofilm Formation
- PMID: 39409056
- PMCID: PMC11476977
- DOI: 10.3390/ijms251910727
Pentadecanoic Acid-Releasing PDMS: Towards a New Material to Prevent S. epidermidis Biofilm Formation
Abstract
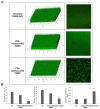
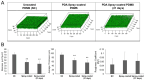
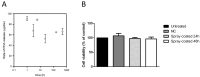
Microbial biofilm formation on medical devices paves the way for device-associated infections. Staphylococcus epidermidis is one of the most common strains involved in such infections as it is able to colonize numerous devices, such as intravenous catheters, prosthetic joints, and heart valves. We previously reported the antibiofilm activity against S. epidermidis of pentadecanoic acid (PDA) deposited by drop-casting on the silicon-based polymer poly(dimethyl)siloxane (PDMS). This material exerted an antibiofilm activity by releasing PDA; however, a toxic effect on bacterial cells was observed, which could potentially favor the emergence of resistant strains. To develop a PDA-functionalized material for medical use and overcome the problem of toxicity, we produced PDA-doped PDMS by either spray-coating or PDA incorporation during PDMS polymerization. Furthermore, we created a strategy to assess the kinetics of PDA release using ADIFAB, a very sensitive free fatty acids fluorescent probe. Spray-coating resulted in the most promising strategy as the concentration of released PDA was in the range 0.8-1.5 μM over 21 days, ensuring long-term effectiveness of the antibiofilm molecule. Moreover, the new coated material resulted biocompatible when tested on immortalized human keratinocytes. Our results indicate that PDA spray-coated PDMS is a promising material for the production of medical devices endowed with antibiofilm activity.
Keywords: ABIFAB; PDMS; S. epidermidis; antibiofilm; pentadecanoic acid; surface coating.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

