Platelet Proteomics and Tissue Metabolomics Investigation for the Mechanism of Aspirin Eugenol Ester on Preventive Thrombosis Mechanism in a Rat Thrombosis Model
- PMID: 39409077
- PMCID: PMC11476519
- DOI: 10.3390/ijms251910747
Platelet Proteomics and Tissue Metabolomics Investigation for the Mechanism of Aspirin Eugenol Ester on Preventive Thrombosis Mechanism in a Rat Thrombosis Model
Abstract
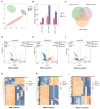
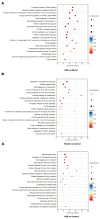
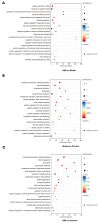
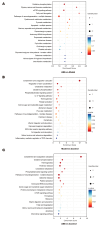
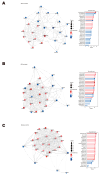
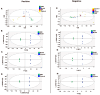
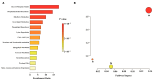
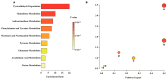
Platelet activation is closely related to thrombosis. Aspirin eugenol ester (AEE) is a novel medicinal compound synthesized by esterifying aspirin with eugenol using the pro-drug principle. Pharmacological and pharmacodynamic experiments showed that AEE has excellent anti-inflammatory, antioxidant, and inhibitory platelet activation effects, preventing thrombosis. However, the regulatory network and action target of AEE in inhibiting platelet activation remain unknown. This study aimed to investigate the effects of AEE on platelets of thrombosed rats to reveal its regulatory mechanism via a multi-omics approach. The platelet proteomic results showed that 348 DEPs were identified in the AEE group compared with the model group, of which 87 were up- and 261 down-regulated. The pathways in this result were different from previous results, including mTOR signaling and ADP signaling at P2Y purinoceptor 12. The metabolomics of heart and abdominal aortic tissue results showed that the differential metabolites were mainly involved in steroid biosynthesis, the citric acid cycle, phenylalanine metabolism, phenylalanine, tyrosine, and tryptophan biosynthesis, and glutathione metabolism. Molecular docking results showed that AEE had a better binding force to both the COX-1 and P2Y12 protein. AEE could effectively inhibit platelet activation by inhibiting COX-1 protein and P2Y12 protein activity, thereby inhibiting platelet aggregation. Therefore, AEE can have a positive effect on inhibiting platelet activation.
Keywords: aspirin eugenol ester (AEE); metabolomics; molecular docking; platelet; proteomics; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Roth G.A., Mensah G.A., Johnson C.O., Addolorato G., Ammirati E., Baddour L.M., Barengo N.C., Beaton A.Z., Benjamin E.J., Benziger C.P., et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll of Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. - DOI - PMC - PubMed
-
- Thon J.N., Italiano J.E. Handbook of Experimental Pharmacology. Springer; Berlin/Heidelberg, Germany: 2012. Platelets: Production, morphology and ultrastructure; pp. 3–22. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

