Cannabinoids and Genetic Epilepsy Models: A Review with Focus on CDKL5 Deficiency Disorder
- PMID: 39409097
- PMCID: PMC11476665
- DOI: 10.3390/ijms251910768
Cannabinoids and Genetic Epilepsy Models: A Review with Focus on CDKL5 Deficiency Disorder
Abstract
Pediatric genetic epilepsies, such as CDKL5 Deficiency Disorder (CDD), are severely debilitating, with early-onset seizures occurring more than ten times daily in extreme cases. Existing antiseizure drugs frequently prove ineffective, which significantly impacts child development and diminishes the quality of life for patients and caregivers. The relaxation of cannabis legislation has increased research into potential therapeutic properties of phytocannabinoids such as cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). CBD's antiseizure properties have shown promise, particularly in treating drug-resistant genetic epilepsies associated with Lennox-Gastaut syndrome (LGS), Dravet syndrome (DS), and Tuberous Sclerosis Complex (TSC). However, specific research on CDD remains limited. Much of the current evidence relies on anecdotal reports of artisanal products lacking accurate data on cannabinoid composition. Utilizing model systems like patient-derived iPSC neurons and brain organoids allows precise dosing and comprehensive exploration of cannabinoids' pharmacodynamics. This review explores the potential of CBD, THC, and other trace cannabinoids in treating CDD and focusing on clinical trials and preclinical models to elucidate the cannabinoid's potential mechanisms of action in disrupted CDD pathways and strengthen the case for further research into their potential as anti-epileptic drugs for CDD. This review offers an updated perspective on cannabinoid's therapeutic potential for CDD.
Keywords: CBD; CDD; CDKL5; cannabidiol; cannabinoids; refractory epilepsy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
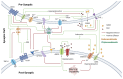

References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- NA/State Government of Victoria's Operational Infrastructure Support Program
- 2018-16/Foundation for Children Project Grant
- Strategic Pilot Project in Stem Cell and Genomic Medicine Research Grant/Murdoch Children's Research Institute
- MDBR-20-106-CDKL5/Million Dollar Bike Ride pilot grant from the Orphan Disease Center of the University of Pennsylvania
- NA/CDKL5 Forum Junior Fellowship from the Loulou Foundation
- MRF2007465/Medical Research Future Funds (MRFF) Stem Cell Therapies Mission
- Near Miss grant/Murdoch Children's Research Institute
- trategic Pilot Project in Stem Cell and Genomics Medicine/Murdoch Children's Research Institute
- Near Miss Pilot Award/VESKI
- Chair in Genomic Medicine/Royal Children's Hospital Foundation
LinkOut - more resources
Full Text Sources

