Descriptive Analysis of Common Fusion Mutations in Papillary Thyroid Carcinoma in Hungary
- PMID: 39409115
- PMCID: PMC11477448
- DOI: 10.3390/ijms251910787
Descriptive Analysis of Common Fusion Mutations in Papillary Thyroid Carcinoma in Hungary
Abstract
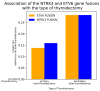
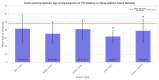
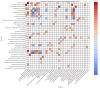
Thyroid cancer is the most common type of endocrine malignancy. Papillary thyroid carcinoma (PTC) is its predominant subtype, which is responsible for the vast majority of cases. It is true that PTC is a malignant tumor with a very good prognosis due to effective primary therapeutic approaches such as thyroidectomy and radioiodine (RAI) therapy. However, we are often required to indicate second-line treatments to eradicate the tumor properly. In these scenarios, molecular therapies are promising alternatives, especially if specifically targetable mutations are present. Many of these targetable gene alterations originate from gene fusions, which can be found using molecular diagnostics like next-generation sequencing (NGS). Nonetheless, molecular profiling is far from being a routine procedure in the initial phase of PTC diagnostics. As a result, the mutation status, except for BRAF V600E mutation, is not included in risk classification algorithms either. This study aims to provide a comprehensive analysis of fusion mutations in PTC and their associations with clinicopathological variables in order to underscore certain clinical settings when molecular diagnostics should be considered earlier, and to demonstrate yet unknown molecular-clinicopathological connections. We conducted a retrospective fusion mutation screening in formalin-fixed paraffin-embedded (FFPE) PTC tissue samples of 100 patients. After quality evaluation by an expert pathologist, RNA isolation was performed, and then NGS was applied to detect 23 relevant gene fusions in the tumor samples. Clinicopathological data were collected from medical and histological records. To obtain the most associations from the multivariate dataset, we used the d-correlation method for our principal component analysis (PCA). Further statistical analyses, including Chi-square tests and logistic regressions, were performed to identify additional significant correlations within certain subsets of the data. Fusion mutations were identified in 27% of the PTC samples, involving nine distinct genes: RET, NTRK3, CCDC6, ETV6, MET, ALK, NCOA4, EML4, and SQSTM1. RET and CCDC6 fusions were associated with type of thyroidectomy, RAI therapy, smaller tumor size, and history of Hashimoto's disease. NCOA4 fusion correlated with sex, multifocality, microcarcinoma character, history of goiter, and obstructive pulmonary disease. EML4 fusion was also linked with surgical procedure type and smaller tumor size, as well as the history of hypothyroidism. SQSTM1 fusion was associated with multifocality and a medical history of thyroid/parathyroid adenoma. NTRK3 and ETV6 fusions showed significant associations with Hashimoto's disease, and ETV6, also with endometriosis. Moreover, fusion mutations were linked to younger age at the time of diagnosis, particularly the fusion of ETV6. The frequent occurrence of fusion mutations and their associations with certain clinicopathological metrics highlight the importance of integrating molecular profiling into routine PTC management. Early detection of fusion mutations can inform surgical decisions and therapeutic strategies, potentially improving clinical outcomes.
Keywords: clinicopathological associations; fusion mutations; gene fusions; molecular diagnostics; papillary thyroid carcinoma; thyroid cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- World Cancer Research Fund International. [(accessed on 31 August 2024)]. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data.
-
- Fathimabeebi P., Noor Al B., Hilal Al M. Epidemiology of Thyroid Cancer in Oman. Ann. Endocrinol. Metab. 2017;1:11–17. doi: 10.36959/433/561. - DOI
-
- National Cancer Institute. [(accessed on 31 August 2024)]; Available online: https://seer.cancer.gov/statfacts/html/thyro.html.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

