Photodynamic Therapy against Colorectal Cancer Using Porphin-Loaded Arene Ruthenium Cages
- PMID: 39409175
- PMCID: PMC11476664
- DOI: 10.3390/ijms251910847
Photodynamic Therapy against Colorectal Cancer Using Porphin-Loaded Arene Ruthenium Cages
Abstract
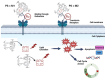
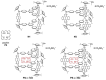
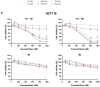
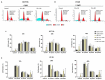
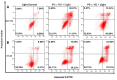
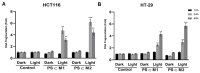
Colorectal cancer (CRC) is the third most common cancer in the world, with an ongoing rising incidence. Despite secure advancements in CRC treatments, challenges such as side effects and therapy resistance remain to be addressed. Photodynamic therapy (PDT) emerges as a promising modality, clinically used in treating different diseases, including cancer. Among the main challenges with current photosensitizers (PS), hydrophobicity and low selective uptake by the tumor remain prominent. Thus, developing an optimal design for PS to improve their solubility and enhance their selective accumulation in cancer cells is crucial for enhancing the efficacy of PDT. Targeted photoactivation triggers the production of reactive oxygen species (ROS), which promote oxidative stress within cancer cells and ultimately lead to their death. Ruthenium (Ru)-based compounds, known for their selective toxicity towards cancer cells, hold potential as anticancer agents. In this study, we investigated the effect of two distinct arene-Ru assemblies, which lodge porphin PS in their inner cavity, and tested them as PDT agents on the HCT116 and HT-29 human CRC cell lines. The cellular internalization of the porphin-loaded assemblies was confirmed by fluorescence microscopy. Additionally, significant photocytotoxicity was observed in both cell lines after photoactivation of the porphin in the cage systems, inducing apoptosis through caspase activation and cell cycle progression disruptions. These findings suggest that arene-Ru assemblies lodging porphin PS are potent candidates for PDT of CRC.
Keywords: apoptosis; arene-ruthenium assemblies; colorectal cancer; photodynamic therapy; photosensitizers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

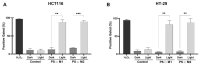



References
-
- Negarandeh R., Salehifar E., Saghafi F., Jalali H., Janbabaei G., Abdhaghighi M.J., Nosrati A. Evaluation of Adverse Effects of Chemotherapy Regimens of 5-Fluoropyrimidines Derivatives and Their Association with DPYD Polymorphisms in Colorectal Cancer Patients. BMC Cancer. 2020;20:560. doi: 10.1186/s12885-020-06904-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

