A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy
- PMID: 39409825
- PMCID: PMC11475781
- DOI: 10.3390/ani14192876
A20 Alleviates the Inflammatory Response in Bovine Endometrial Epithelial Cells by Promoting Autophagy
Abstract
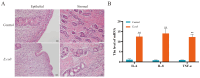
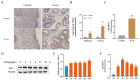
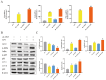
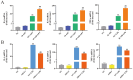
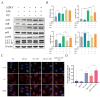
Endometritis represents a prevalent condition in perinatal dairy cows. Bovine endometrial epithelial cells (BEECs), as the primary interface between cavity and the external environment, are particularly vulnerable to infection by pathogenic bacteria following parturition. A20 is essential for regulating inflammation and modulating immune responses. Nevertheless, the exact role of A20 in the BEECs in response to inflammatory response is not fully understood. An endometritis model infected by Escherichia coli (E. coli) in vivo and a BEECs inflammation model induced with lipopolysaccharide (LPS) in vitro were built to investigate the function and governing mechanisms of A20 in endometritis. The results showed that infection with E. coli resulted in endometrial damage, inflammatory cell infiltration, and upregulation of inflammatory factors in dairy cows. Furthermore, A20 expression was upregulated in the endometrium of cows with endometritis and in BEECs following LPS stimulation. A20 overexpression attenuated the level of proinflammatory cytokines in LPS-stimulated BEECs; conversely, A20 knockdown lead to an exacerbated response to LPS stimulation. The overexpression of A20 was shown to activate autophagy and suppress the NF-κB signaling pathway in LPS-stimulated BEECs. However, blocking autophagy with chloroquine notably attenuated the anti-inflammatory effect of A20, leading to the activation of the NF-κB signaling pathway. In summary, the study demonstrated that A20's suppression of inflammation in LPS-stimulated BEECs is associated with the activation of autophagy. Therefore, the A20 protein showed potential as a novel treatment focus for managing endometritis in dairy cows.
Keywords: A20; LPS; autophagy; bovine endometrial epithelial cells; inflammatory response.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Piersanti R.L., Zimpel R., Molinari P.C.C., Dickson M.J., Ma Z., Jeong K.C., Santos J.E.P., Sheldon I.M., Bromfield J.J. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J. Dairy Sci. 2019;102:2686–2697. doi: 10.3168/jds.2018-15595. - DOI - PMC - PubMed
-
- Wu Y., Chen J., Sun Y., Dong X., Wang Z., Chen J., Dong G. PGN and LTA from Staphylococcus aureus Induced Inflammation and Decreased Lactation through Regulating DNA Methylation and Histone H3 Acetylation in Bovine Mammary Epithelial Cells. Toxins. 2020;12:238. doi: 10.3390/toxins12040238. - DOI - PMC - PubMed
Grants and funding
- 32102735, 32072937/National Natural Science Foundation of China
- BK20210808/Natural Science Foundation of Jiangsu Province
- JATS[2023]456/the Earmarked Fund for Jiangsu Agricultural Industry Technology System
- 333/the 333 High-level Talent Training Project of Jiangsu Province (CN)
- No. 8/the International Research Laboratory of Prevention and Control of Important Animal Infectious Diseases and Zoonotic Diseases of Jiangsu Higher Education Institutions
LinkOut - more resources
Full Text Sources

