Altered Gut Microbiome Composition in Dogs with Hyperadrenocorticism: Key Bacterial Genera Analysis
- PMID: 39409832
- PMCID: PMC11476382
- DOI: 10.3390/ani14192883
Altered Gut Microbiome Composition in Dogs with Hyperadrenocorticism: Key Bacterial Genera Analysis
Abstract
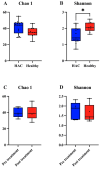
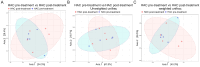
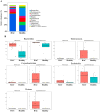
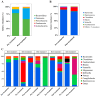
Hyperadrenocorticism (HAC) is a common endocrine disorder in dogs, which is associated with diverse metabolic abnormalities. We hypothesized that elevated cortisol levels in dogs with HAC disrupt the gut microbiome (GM), and this disruption persists even after trilostane treatment. This study explored GM composition in dogs with HAC. We included 24 dogs, 15 with HAC and 9 healthy controls, and followed up with 5 dogs with HAC who received trilostane treatment. The GM analysis revealed significant compositional changes in dogs with HAC, including reduced microbiome diversity compared to healthy controls, particularly in rare taxa, as indicated by the Shannon index (p = 0.0148). Beta diversity analysis further showed a distinct clustering of microbiomes in dogs with HAC, separating them from healthy dogs (p < 0.003). Specifically, an overrepresentation of Proteobacteria (Pseudomonadota), Actinobacteria, Bacteroides, Enterococcus, Corynebacterium, Escherichia, and Proteus populations occurred alongside a decreased Firmicutes (Bacillota) population. Despite trilostane treatment, gut dysbiosis persisted in dogs with HAC at a median of 41 d post treatment, suggesting its potential role in ongoing metabolic issues. We identified GM dysbiosis in dogs with HAC by examining key bacterial genera, offering insights into potential interventions like probiotics or fecal microbiota transplants for better HAC management.
Keywords: cortisol; dysbiosis; hyperadrenocorticism; hypercortisolism; microbiome; trilostane.
Conflict of interest statement
S.-M.K. was employed by KR Lab Bio Incorporation. The authors declare that they have no financial or personal relationships with any individuals or organizations that could influence or bias the content of the paper. The funders had no role in the study design, collection, analyses, or interpretation of data; in writing the manuscript; or in the decision to publish the results.
Figures




References
-
- Pérez Alenza M.D., Melian C. Hyperadrenocorticism in Dogs. In: Ettinger S.J., Feldman E.C., Cote E., editors. Textbook of Veterinary Internal Medicine. Volume 2. Elsevier; Maryland Heights, MO, USA: 2017. pp. 4345–4389.
-
- Valassi E., Manichanh C., Amodru V., Fernández P.G., Gaztambide S., Yañez F., Martel-Duguech L., Puig-Domingo M., Webb S.M. Gut Microbial Dysbiosis in Patients with Cushing’s Disease in Long-Term Remission. Relationship with Cardiometabolic Risk. Front. Endocrinol. 2023;14:1074757. doi: 10.3389/fendo.2023.1074757. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

