Morphology of Larger Salivary Glands in Peccaries (Pecari tajacu Linnaeus, 1758)
- PMID: 39409840
- PMCID: PMC11475750
- DOI: 10.3390/ani14192891
Morphology of Larger Salivary Glands in Peccaries (Pecari tajacu Linnaeus, 1758)
Abstract
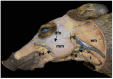
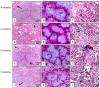
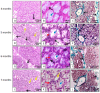
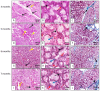
This work aims to study the major salivary gland morphology of peccaries during their growth. The glands were analyzed using macroscopic description, light microscopy, electron microscopy, histochemistry, and immunohistochemistry. Topographically, the salivary glands resemble other animals, including domestic animals and pigs. During growth, the parotid enlarges and mandibular gland loses weight. Histologically, the parotid has serous production, and sublingual has mucous production, resembles most species, however, mandibular gland produces mucous, unlike other animals, including pigs, which produce seromucous secretion. Histochemically, parotid produces more acidic mucins than pigs and it undergoes maturation during development; mandibular, and especially the sublingual gland, produce more acidic and basic mucopolysaccharides than pigs. The results found with transmission and scanning electron microscopy techniques corroborate the histological and histochemistry findings. The major salivary glands were positive to different lecithins (Com-A, BSA-I-B4, WGA and PNA), which were also more positive than in pigs and sheep. We conclude that collared peccaries have a salivary secretion that facilitates the digestion of carbohydrates, and biometric characteristics and positivity to lecithins that facilitate adaptation to foods with antinutritional factors.
Keywords: Tayassuidae; lecithins; ultrastructure; wild animals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Denny P., Hagen F.K., Hardt M., Liao L., Yan W., Arellanno M., Bassilian S., Bedi G.S., Boontheung P., Cociorva D., et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome Res. 2008;7:1994–2006. doi: 10.1021/pr700764j. - DOI - PMC - PubMed
-
- Oliveira Júnior C.M., Bezerra F.V.F., Câmara F.V., Vale A.M., Oliveira G.B., Silva A.R., Ambrosio C.E., Oliveira M.F. Morfologia das glândulas salivares maiores em cutias (Dasyprocta leporina Linnaeus, 1766) Pesqui. Veterinária Bras. 2016;36:227–236. doi: 10.1590/S0100-736X2016000300013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

