Xeroderma Pigmentosum Type C Primary Skin Fibroblasts Overexpress HGF and Promote Squamous Cell Carcinoma Invasion in the Absence of Genotoxic Stress
- PMID: 39409898
- PMCID: PMC11475422
- DOI: 10.3390/cancers16193277
Xeroderma Pigmentosum Type C Primary Skin Fibroblasts Overexpress HGF and Promote Squamous Cell Carcinoma Invasion in the Absence of Genotoxic Stress
Abstract
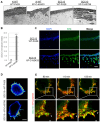
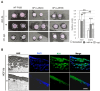
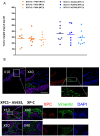
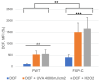
Xeroderma pigmentosum (XP) is a very rare recessive disease caused by the incapacity to resolve ultraviolet-induced DNA lesions through Nucleotide Excision Repair (NER). Most XP patients suffer from aggressive skin carcinoma and melanoma at a very early age (<8). Our previous results showed that primary XP fibroblasts isolated from healthy (non-photo-exposed) skin negatively impact the extracellular matrix and fail to activate the innate immune system. Here, we show for the first time that XP-C fibroblasts also play a major role in cancer cell invasion ex vivo and in vivo through the overexpression of Hepatocyte Growth Factor/Scatter Factor (HGF/SF) in the absence of genotoxic attacks. The use of inhibitors of the activation of the HGF/SF pathway counteracted the effects of XP fibroblasts on the growth of cancer cells, suggesting new perspectives in the care of XP patients.
Keywords: HGF/SF; fibroblasts; squamous cell carcinoma; xeroderma pigmentosum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Overexpression of matrix metalloproteinase 1 in dermal fibroblasts from DNA repair-deficient/cancer-prone xeroderma pigmentosum group C patients.Oncogene. 2008 Sep 4;27(39):5223-32. doi: 10.1038/onc.2008.153. Epub 2008 May 12. Oncogene. 2008. PMID: 18469853
-
Xeroderma Pigmentosum C (XPC) Mutations in Primary Fibroblasts Impair Base Excision Repair Pathway and Increase Oxidative DNA Damage.Front Genet. 2020 Nov 27;11:561687. doi: 10.3389/fgene.2020.561687. eCollection 2020. Front Genet. 2020. PMID: 33329698 Free PMC article.
-
Comparative study of nucleotide excision repair defects between XPD-mutated fibroblasts derived from trichothiodystrophy and xeroderma pigmentosum patients.DNA Repair (Amst). 2008 Dec 1;7(12):1990-8. doi: 10.1016/j.dnarep.2008.08.009. Epub 2008 Oct 10. DNA Repair (Amst). 2008. PMID: 18817897
-
Sunlight, Vitamin D, and Xeroderma Pigmentosum.Adv Exp Med Biol. 2020;1268:319-331. doi: 10.1007/978-3-030-46227-7_16. Adv Exp Med Biol. 2020. PMID: 32918226 Review.
-
Xeroderma pigmentosum: from symptoms and genetics to gene-based skin therapy.Cells Tissues Organs. 2004;177(3):189-98. doi: 10.1159/000079993. Cells Tissues Organs. 2004. PMID: 15388993 Review.
References
-
- Bradford P.T., Goldstein A.M., Tamura D., Khan S.G., Ueda T., Boyle J., Oh K.S., Imoto K., Inui H., Moriwaki S., et al. Cancer and neurologic degeneration in xeroderma pigmentosum: Long term follow-up characterises the role of DNA repair. J. Med. Genet. 2011;48:168–176. doi: 10.1136/jmg.2010.083022. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

