Impact of Optimized Ku-DNA Binding Inhibitors on the Cellular and In Vivo DNA Damage Response
- PMID: 39409907
- PMCID: PMC11475570
- DOI: 10.3390/cancers16193286
Impact of Optimized Ku-DNA Binding Inhibitors on the Cellular and In Vivo DNA Damage Response
Abstract
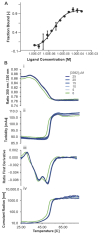
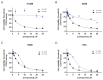
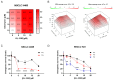
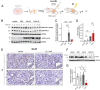
Background: DNA-dependent protein kinase (DNA-PK) is a validated cancer therapeutic target involved in DNA damage response (DDR) and non-homologous end-joining (NHEJ) repair of DNA double-strand breaks (DSBs). Ku serves as a sensor of DSBs by binding to DNA ends and activating DNA-PK. Inhibition of DNA-PK is a common strategy to block DSB repair and improve efficacy of ionizing radiation (IR) therapy and radiomimetic drug therapies. We have previously developed Ku-DNA binding inhibitors (Ku-DBis) that block in vitro and cellular NHEJ activity, abrogate DNA-PK autophosphorylation, and potentiate cellular sensitivity to IR. Results and Conclusions: Here we report the discovery of oxindole Ku-DBis with improved cellular uptake and retained potent Ku-inhibitory activity. Variable monotherapy activity was observed in a panel of non-small cell lung cancer (NSCLC) cell lines, with ATM-null cells being the most sensitive and showing synergy with IR. BRCA1-deficient cells were resistant to single-agent treatment and antagonistic when combined with DSB-generating therapies. In vivo studies in an NSCLC xenograft model demonstrated that the Ku-DBi treatment blocked IR-dependent DNA-PKcs autophosphorylation, modulated DDR, and reduced tumor cell proliferation. This represents the first in vivo demonstration of a Ku-targeted DNA-binding inhibitor impacting IR response and highlights the potential therapeutic utility of Ku-DBis for cancer treatment.
Keywords: DNA-PK; Ku-DBis; double-strand break repair; non-homologous end joining; non-small cell lung cancer; small-molecule inhibitors.
Conflict of interest statement
Author Karim Ben Ali Gacem was employed by the company Sanofi. J.J. Turchi is a co-founder and CSO of NERx Biosciences. J.J. Turchi and N.S. Gavande are co-inventors listed on patents covering the compounds described in this manuscript. All other authors declare no potential conflicts of interest.
Figures


References
-
- Gavande N.S., VanderVere-Carozza P.S., Pawelczak K.S., Mendoza-Munoz P., Vernon T.L., Hanakahi L.A., Summerlin M., Dynlacht J.R., Farmer A.H., Sears C.R., et al. Discovery and development of novel DNA-PK inhibitors by targeting the unique Ku-DNA interaction. Nucleic Acids Res. 2020;48:11536–11550. doi: 10.1093/nar/gkaa934. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

