The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy
- PMID: 39409932
- PMCID: PMC11476037
- DOI: 10.3390/cancers16193312
The Association between Sampling and Survival in Patients with Pancreatic Ductal Adenocarcinoma Who Received Neoadjuvant Therapy and Pancreaticoduodenectomy
Abstract
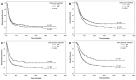
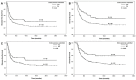
Adequate sampling is essential to an accurate pathologic evaluation of pancreatectomy specimens resected for pancreatic ductal adenocarcinoma (PDAC) after neoadjuvant therapy (NAT). However, limited data are available for the association between the sampling and survival in these patients. We examined the association of the entire submission of the tumor (ESOT) and the entire submission of the pancreas (ESOP) with disease-free survival (DFS) and overall survival (OS), as well as their correlations with clinicopathologic features, for 627 patients with PDAC who received NAT and pancreaticoduodenectomy. We demonstrated that both ESOT and ESOP were associated with lower ypT, less frequent perineural invasion, and better tumor response (p < 0.05). ESOP was also associated with a smaller tumor size (p < 0.001), more lymph nodes (p < 0.001), a lower ypN stage (p < 0.001), better differentiation (p = 0.02), and less frequent lymphovascular invasion (p = 0.009). However, since ESOP and ESOT were primarily conducted for cases with no grossly identifiable tumor or minimal residual carcinoma in initial sections, potential bias cannot be excluded. Both ESOT and ESOP were associated with less frequent recurrence/metastasis and better DFS and OS (p < 0.05) in the overall study population. ESOP was associated with better DFS and better OS in patients with ypT0/ypT1 or ypN0 tumors and better OS in patients with complete or near-complete response (p < 0.05). ESOT was associated with better OS in patients with ypT0/ypT1 or ypN0 tumors (p < 0.05). Both ESOT and ESOP were independent prognostic factors for OS according to multivariate survival analyses. Therefore, accurate pathologic evaluation using ESOP and ESOT is associated with the prognosis in PDAC patients with complete or near-complete pathologic response and ypT0/ypT1 tumor after NAT.
Keywords: neoadjuvant therapy; pancreatic cancer; sampling; survival.
Conflict of interest statement
The authors declare no conflicts of interest pertinent to this study.
Figures


References
-
- Jang J.Y., Han Y., Lee H., Kim S.W., Kwon W., Lee K.H., Oh D.Y., Chie E.K., Lee J.M., Heo J.S., et al. Oncological Benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront Surgery in Patients with Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann. Surg. 2018;268:215–222. doi: 10.1097/SLA.0000000000002705. - DOI - PubMed
-
- Reni M., Balzano G., Zanon S., Zerbi A., Rimassa L., Castoldi R., Pinelli D., Mosconi S., Doglioni C., Chiaravalli M., et al. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): A randomised, open-label, phase 2–3 trial. Lancet Gastroenterol. Hepatol. 2018;3:413–423. doi: 10.1016/S2468-1253(18)30081-5. - DOI - PubMed
-
- Versteijne E., Suker M., Groothuis K., Akkermans-Vogelaar J.M., Besselink M.G., Bonsing B.A., Buijsen J., Busch O.R., Creemers G.M., van Dam R.M., et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J. Clin. Oncol. 2020;38:1763–1773. doi: 10.1200/JCO.19.02274. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

