Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis
- PMID: 39409953
- PMCID: PMC11482613
- DOI: 10.3390/cancers16193331
Demographic and Clinical Characteristics of Malignant Solitary Fibrous Tumors: A SEER Database Analysis
Abstract
Background/objectives: Solitary fibrous tumors (SFTs) represent a rare mesenchymal malignancy that can occur anywhere in the body. Due to the low prevalence of the disease, there is a lack of contemporary data regarding patient demographics and cancer-control outcomes.
Methods: Within the SEER database (2000-2019), we identified 1134 patients diagnosed with malignant SFTs. The distributions of patient demographics and tumor characteristics were tabulated. Cumulative incidence plots and competing risks analyses were used to estimate cancer-specific mortality (CSM) after adjustment for other-cause mortality.
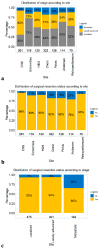
Results: Of 1134 SFT patients, 87% underwent surgical resection. Most of the tumors were in the chest (28%), central nervous system (22%), head and neck (11%), pelvis (11%), extremities (10%), abdomen (10%) and retroperitoneum (6%), in that order. Stage was distributed as follows: localized (42%) vs. locally advanced (35%) vs. metastatic (13%). In multivariable competing risks models, independent predictors of higher CSM were stage (locally advanced HR: 1.6; metastatic HR: 2.9), non-surgical management (HR: 3.6) and tumor size (9-15.9 cm HR: 1.6; ≥16 cm HR: 1.9).
Conclusions: We validated the importance of stage and surgical resection as independent predictors of CSM in malignant SFTs. Moreover, we provide novel observations regarding the independent importance of tumor size, regardless of the site of origin, stage and/or surgical resection status.
Keywords: competing risks analyses; solitary fibrous tumor; tumor-size cut-offs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Machado I., Giner F., Cruz J., Lavernia J., Marhuenda-Fluixa A., Claramunt R., López-Guerrero J.A., Navarro S., Ferrandez A., Bujeda Á.B., et al. Extra-Meningeal Solitary Fibrous Tumor: An Evolving Entity with Chameleonic Morphological Diversity, a Hallmark Molecular Alteration and Unresolved Issues in Risk Stratification Assessment. Histol. Histopathol. 2023;38:1079–1097. doi: 10.14670/HH-18-608. - DOI - PubMed

