Advancement and Challenges in Monitoring of CAR-T Cell Therapy: A Comprehensive Review of Parameters and Markers in Hematological Malignancies
- PMID: 39409959
- PMCID: PMC11475293
- DOI: 10.3390/cancers16193339
Advancement and Challenges in Monitoring of CAR-T Cell Therapy: A Comprehensive Review of Parameters and Markers in Hematological Malignancies
Abstract
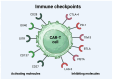
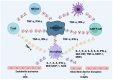
Chimeric antigen receptor T-cell (CAR-T) therapy has revolutionized the treatment for relapsed/refractory B-cell lymphomas. Despite its success, this therapy is accompanied by a significant frequency of adverse events, including cytokine release syndrome (CRS), immune-effector-cell-associated neurotoxicity syndrome (ICANS), or cytopenias, reaching even up to 80% of patients following CAR-T cell therapy. CRS results from the uncontrolled overproduction of proinflammatory cytokines, which leads to symptoms such as fever, headache, hypoxia, or neurological complications. CAR-T cell detection is possible by the use of flow cytometry (FC) or quantitative polymerase chain reaction (qPCR) assays, the two primary techniques used for CAR-T evaluation in peripheral blood, bone marrow (BM), and cerebrospinal fluid (CSF). State-of-the-art imaging technologies play a crucial role in monitoring the distribution and persistence of CAR-T cells in clinical trials. Still, they can also be extended with the use of FC and digital PCR (dPCR). Monitoring the changes in cell populations during disease progression and treatment gives an important insight into how the response to CAR-T cell therapy develops on a cellular level. It can help improve the therapeutic design and optimize CAR-T cell therapy to make it more precise and personalized, which is crucial to overcoming the problem of tumor relapse.
Keywords: biomarkers; chimeric antigen receptor T-cell (CAR-T); flow cytometry (FC); immunotherapy; monitoring; polymerase chain reaction (PCR).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Hayden P.J., Roddie C., Bader P., Basak G.W., Bonig H., Bonini C., Chabannon C., Ciceri F., Corbacioglu S., Ellard R., et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA) Ann. Oncol. 2022;33:259–275. doi: 10.1016/j.annonc.2021.12.003. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

