Rare Oncogenic Fusions in Pediatric Central Nervous System Tumors: A Case Series and Literature Review
- PMID: 39409964
- PMCID: PMC11475864
- DOI: 10.3390/cancers16193344
Rare Oncogenic Fusions in Pediatric Central Nervous System Tumors: A Case Series and Literature Review
Abstract
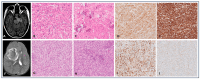
Background and Objectives: Central Nervous System (CNS) pediatric tumors represent the most common solid tumors in children with a wide variability in terms of survival and therapeutic response. By contrast to their adult counterpart, the mutational landscape of pediatric CNS tumors is characterized by oncogenic fusions rather than multiple mutated genes. CNS pediatric tumors associated with oncogenic fusions represent a complex landscape of tumors with wide radiological, morphological and clinical heterogeneity. In the fifth CNS WHO classification, there are few pediatric CNS tumors for which diagnosis is based on a single oncogenic fusion. This work aims to provide an overview of the impact of rare oncogenic fusions (NTRK, ROS, ALK, MET, FGFR, RAF, MN1, BCOR and CIC genes) on pathogenesis, histological phenotype, diagnostics and theranostics in pediatric CNS tumors. We report four cases of pediatric CNS tumors associated with NTRK (n = 2), ROS (n = 1) and FGFR3 (n = 1) oncogenic fusion genes as a proof of concept. Cases presentation and literature review: The literature review and the cohort that we described here underline that most of these rare oncogenic fusions are not specific to a single morpho-molecular entity. Even within tumors harboring the same oncogenic fusions, a wide range of morphological, molecular and epigenetic entities can be observed. Conclusions: These findings highlight the need for caution when applying the fifth CNS WHO classification, as the vast majority of these fusions are not yet incorporated in the diagnosis, including grade evaluation and DNA methylation classification.
Keywords: CNS WHO classification; DNA methylation; RNA sequencing; oncogenic fusions; pediatric CNS tumors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sahm F., Brandner S., Bertero L., Capper D., French P.J., Figarella-Branger D., Giangaspero F., Haberler C., Hegi M.E., Kristensen B.W., et al. Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline. Neuro Oncol. 2023;25:1731–1749. doi: 10.1093/neuonc/noad100. - DOI - PMC - PubMed
-
- Qaddoumi I., Orisme W., Wen J., Santiago T., Gupta K., Dalton J.D., Tang B., Haupfear K., Punchihewa C., Easton J., et al. Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol. 2016;131:833–845. doi: 10.1007/s00401-016-1539-z. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

