Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer
- PMID: 39409965
- PMCID: PMC11476249
- DOI: 10.3390/cancers16193345
Antibody-Drug Conjugates and Their Potential in the Treatment of Patients with Biliary Tract Cancer
Abstract
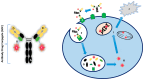
Background: Biliary tract cancers (BTCs) are aggressive in nature, often presenting asymptomatically until they are diagnosed at an advanced stage. Surgical resection or liver transplantation are potential curative options. However, a large proportion of patients present with incurable locally advanced or metastatic disease and most of these patients are only eligible for palliative chemotherapy or best supportive care. More recently, targeted therapies have proven beneficial in a molecularly selected subgroup of patients with cholangiocarcinoma who have progressed on previous lines of systemic treatment. However, only a minority of patients with BTCs whose tumours harbour specific molecular alterations can access these therapies. Methods: In relation to ADCs, studies regarding use of antibody-drug conjugates in cancer, particularly in BTCs, were searched in Embase (1974 to 2024) and Ovid MEDLINE(R) (1946 to 2024) to obtain relevant articles. Examples of current clinical trials utilising ADC treatment in BTCs were extracted from the ClinicalTrials.gov trial registry. Conclusions: Overall, this review has highlighted that ADCs have shown encouraging outcomes in cancer therapy, and this should lead to further research including in BTCs, where treatment options are often limited. The promising results observed with ADCs in various cancers underscore their potential as a transformative approach in oncology, warranting continued exploration and development and the need for education on the management of their specific toxicities. By addressing current challenges and optimising ADC design and application, future studies could potentially improve treatment outcomes for patients with BTCs and beyond, potentially in both early and advanced stage settings.
Keywords: anti-HER2; antibody–drug conjugate; biliary tract cancer; payload; targeted therapy.
Conflict of interest statement
SA. received speaker honoraria from Novartis. MMN. received research grant support from Astra Zeneca, Servier, Ipsen, and NuCana. She received travel and accommodation support from Bayer and Ipsen and speaker honoraria from Pfizer, Ipsen, NuCana, Mylan, and AAA. She served on advisory boards for Celgene, Ipsen, Sirtex, Baxalta, Incyte, and Astra Zeneca, all outside of the scope of this work. R.H. served on advisory boards for Roche, BMS, Eisai, Celgene, Beigene, Ipsen, and BTG. He received speaker fees from Eisai, Ipsen, Mylan, PrimeOncology, and received travel and educational support from Bayer, BMS, and Roche, all outside of the scope of this work. MF. Received honoraria and travel and accommodation support from AAA/Novartis and travel and accommodation support from Roche, all are outside the scope of this work. VF-has received travel and educational support from Servier, Ipse, and AstraZeneca. U.A and T.J declare no conflicts of interest.
Figures

References
-
- Vogel A., Bridgewater J., Edeline J., Kelley R.K., Klümpen H.J., Malka D., Primrose J.N., Rimassa L., Stenzinger A., Valle J.W., et al. Biliary Tract Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023;34:127–140. doi: 10.1016/j.annonc.2022.10.506. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

