A Systematic Review of Phase II/III Trials of Hypofractionated versus Conventionally Fractionated Radiation Therapy in Stage III Non-Small Cell Lung Cancer Patients
- PMID: 39410003
- PMCID: PMC11475879
- DOI: 10.3390/cancers16193384
A Systematic Review of Phase II/III Trials of Hypofractionated versus Conventionally Fractionated Radiation Therapy in Stage III Non-Small Cell Lung Cancer Patients
Abstract
Introduction: This systematic review evaluated whether curative intent hypofractionated radiation therapy improved survival (primary endpoint) as compared to standard conventionally fractionated radiation therapy for stage III non-small cell lung cancer (NSCLC) patients. Toxicity was also examined as a secondary endpoint.
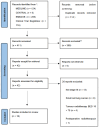
Methods: Electronic bibliographic databases were searched from 1 January 1990 to 31 March 2024. Phase II and phase III trials were included to assess survival (primary outcome) and toxicity (secondary outcome) for newly diagnosed stage III NSCLC patients.
Results: Eight phase II trials (n = 349 participants), 3 randomized phase II trials (n = 382 participants), and 5 randomized phase III trials (n = 811 participants), for a total of 1542 participants, were identified. The published trials were heterogeneous, with a wide variety of dose prescriptions. A wide range of survivals (median survival 13.6 months-42.5 months) and toxicities such as grade 3 or higher esophagitis (0-42%) and grade 3 or higher pneumonitis (0-18%) were reported.
Conclusions: There is no level 1 evidence to date that suggests that any hypofractionated regimen (dose escalated or not) improves survival as compared to conventionally fractionated radiation. The published phase III trials have been powered for superiority (not equivalence) for the hypofractionated arm. Toxicity with hypofractionated regimens may be similar to conventionally fractionated regimens when normal tissue radiotherapy constraints are kept within tolerance limits. It is unclear how the use of systemic therapy may negatively affect radiation toxicity with hypofractionated radiation therapy.
Keywords: hypofractionated; lung cancer; radiation; systematic review.
Conflict of interest statement
May N. Tsao: none. Alexander V. Louie received honoraria from Astra-Zeneca for speaker’s fees and advisory board participation. Patrick Cheung: none. Ian Poon is a member of the advisory board for Sanofi Aventis and Astra-Zeneca. Yee Ung: none.
Figures
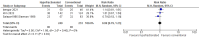
Similar articles
-
External beam radiation dose escalation for high grade glioma.Cochrane Database Syst Rev. 2020 May 21;5(5):CD011475. doi: 10.1002/14651858.CD011475.pub3. Cochrane Database Syst Rev. 2020. PMID: 32437039 Free PMC article.
-
Accelerated hypofractionated three-dimensional conformal radiation therapy (3 Gy/fraction) combined with concurrent chemotherapy for patients with unresectable stage III non-small cell lung cancer: preliminary results of an early terminated phase II trial.BMC Cancer. 2016 Apr 23;16:288. doi: 10.1186/s12885-016-2314-1. BMC Cancer. 2016. PMID: 27108080 Free PMC article. Clinical Trial.
-
Accelerated Hypofractionated Image-Guided vs Conventional Radiotherapy for Patients With Stage II/III Non-Small Cell Lung Cancer and Poor Performance Status: A Randomized Clinical Trial.JAMA Oncol. 2021 Oct 1;7(10):1497-1505. doi: 10.1001/jamaoncol.2021.3186. JAMA Oncol. 2021. PMID: 34383006 Free PMC article. Clinical Trial.
-
Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial.Lancet Oncol. 2019 Nov;20(11):1531-1543. doi: 10.1016/S1470-2045(19)30569-8. Epub 2019 Sep 17. Lancet Oncol. 2019. PMID: 31540791 Free PMC article. Clinical Trial.
-
Hypofractionated radiotherapy in non small cell lung cancer: a review of the current literature.Rev Recent Clin Trials. 2010 May;5(2):103-11. doi: 10.2174/157488710791233608. Rev Recent Clin Trials. 2010. PMID: 20423316 Review.
References
-
- Bezjak A., Temin S., Franklin G., Giaccone G., Govindan R., Johnson M.L., Rimner A., Schneider B.J., Strawn J., Azzoli C.G. Definitive and adjuvant radiotherapy in locally advanced non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology evidence-based clinical practice guideline. J. Clin. Oncol. 2015;33:2100–2105. doi: 10.1200/JCO.2014.59.2360. - DOI - PubMed
-
- Perez C.A., Pajak T.F., Rubin P., Simpson J.R., Mohiuddin M., Brady L.W., Perez-Tamayo R., Rotman M. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::AID-CNCR2820591106>3.0.CO;2-Z. - DOI - PubMed
-
- Perez C.A., Stanley K., Rubin P., Kramer S., Brady L., Perez-Tamayo R., Brown G.S., Concannon J., Rotman M., Seydel H.G. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–2753. doi: 10.1002/1097-0142(19800601)45:11<2744::AID-CNCR2820451108>3.0.CO;2-U. - DOI - PubMed
-
- Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S., Bogart J., Hu C., Forster K., Magliocco A., et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

