Neoadjuvant Pembrolizumab Plus Chemotherapy in Early-Stage Triple-Negative Breast Cancer: A Nationwide Retrospective Turkish Oncology Group Study
- PMID: 39410009
- PMCID: PMC11475936
- DOI: 10.3390/cancers16193389
Neoadjuvant Pembrolizumab Plus Chemotherapy in Early-Stage Triple-Negative Breast Cancer: A Nationwide Retrospective Turkish Oncology Group Study
Abstract
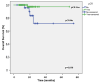
Background/Objectives: Following the results of the phase 3 KEYNOTE-522 trial, the U.S. Food and Drug Administration approved pembrolizumab, a humanized IgG4 kappa monoclonal antibody, in combination with neoadjuvant chemotherapy as a new standard of care for high-risk early-stage triple-negative breast cancer (TNBC). This retrospective, multicenter study in Türkiye assessed the real-world efficacy and safety of neoadjuvant pembrolizumab combined with chemotherapy in early-stage TNBC. Methods: The study included 108 patients treated between 2021 and 2023 across 14 oncology centers. Three distinct neoadjuvant regimens incorporating pembrolizumab were administered at the discretion of the treating physicians. The primary outcomes were the pathological complete response (pCR) rate after neoadjuvant therapy and the 2-year event-free survival (EFS) and overall survival (OS) rates. Results: The observed pCR rate was 63.9%, closely mirroring the 64.8% reported in the KEYNOTE-522 trial. At the two-year mark, the EFS rate was 87.2% and the OS rate was 92.3%. Multivariable analysis identified pCR as the sole independent predictor of both EFS and OS. The safety profile was consistent with previous clinical trial data, with most adverse events being of grade 1-2 in severity. Conclusions: These findings provide valuable real-world confirmation of the efficacy and safety of neoadjuvant pembrolizumab-chemotherapy in early-stage TNBC, complementing evidence from randomized trials.
Keywords: event-free survival; neoadjuvant therapy; overall survival; pathological complete response; pembrolizumab; real world; safety; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources

