Combined PET Radiotracer Approach Reveals Insights into Stromal Cell-Induced Metabolic Changes in Pancreatic Cancer In Vitro and In Vivo
- PMID: 39410013
- PMCID: PMC11475921
- DOI: 10.3390/cancers16193393
Combined PET Radiotracer Approach Reveals Insights into Stromal Cell-Induced Metabolic Changes in Pancreatic Cancer In Vitro and In Vivo
Abstract
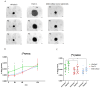
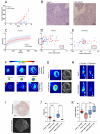
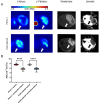
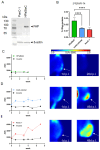
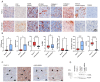
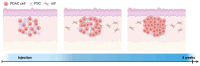
Background/Objective Pancreatic stellate cells (PSCs) in pancreatic adenocarcinoma (PDAC) are producing extracellular matrix, which promotes the formation of a dense fibrotic microenvironment. This makes PDAC a highly heterogeneous tumor-stroma-driven entity, associated with reduced perfusion, limited oxygen supply, high interstitial fluid pressure, and limited bioavailability of therapeutic agents. Methods In this study, spheroid and tumor xenograft models of human PSCs and PanC-1 cells were characterized radiopharmacologically using a combined positron emission tomography (PET) radiotracer approach. [18F]FDG, [18F]FMISO, and [18F]FAPI-74 were employed to monitor metabolic activity, hypoxic metabolic state, and functional expression of fibroblast activation protein alpha (FAPα), a marker of activated PSCs. Results In vitro, PanC-1 and multi-cellular tumor spheroids demonstrated comparable glucose uptake and hypoxia, whereas FAPα expression was significantly higher in PSC spheroids. In vivo, glucose uptake as well as the transition to hypoxia were comparable in PanC-1 and multi-cellular xenograft models. In mice injected with PSCs, FAPα expression decreased over a period of four weeks post-injection, which was attributed to the successive death of PSCs. In contrast, FAPα expression increased in both PanC-1 and multi-cellular xenograft models over time due to invasion of mouse fibroblasts. Conclusion The presented models are suitable for subsequently characterizing stromal cell-induced metabolic changes in tumors using noninvasive molecular imaging techniques.
Keywords: radionuclide theranostics; radiotracers; small animal positron emission tomography; spheroids; tumor microenvironment; xenograft models.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Li C., Morvaridi S., Lam G., Chheda C., Kamata Y., Katsumata M., Edderkaoui M., Yuan X., Nissen N., Pandol S.J., et al. MSP-RON Signaling Is Activated in the Transition From Pancreatic Intraepithelial Neoplasia (PanIN) to Pancreatic Ductal Adenocarcinoma (PDAC) Front. Physiol. 2019;10:147. doi: 10.3389/fphys.2019.00147. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

