Survival of Patients with Metastatic Melanoma Treated with Ipilimumab after PD-1 Inhibitors: A Single-Center Real-World Study
- PMID: 39410017
- PMCID: PMC11475497
- DOI: 10.3390/cancers16193397
Survival of Patients with Metastatic Melanoma Treated with Ipilimumab after PD-1 Inhibitors: A Single-Center Real-World Study
Abstract
Background: When monotherapy with PD-1 inhibitors in metastatic melanoma fails, there are currently no standard second-line choices. In case of the unavailability of clinical trials, ipilimumab represents a possible alternative treatment.
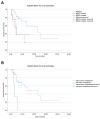
Methods: We collected data of 44 patients who received ipilimumab after the failure of PD-1 inhibitors from July 2017 to May 2023 at our Institute. Overall survival (OS), progression-free survival (PFS), and post-progression survival (PPS) based on BRAF or NRAS mutation status, sex, and the presence of brain metastases were estimated using the Kaplan-Meier method. Cox regression was used to evaluate independence in multivariate analysis. The objective response rate (ORR) was estimated based on RECIST 1.1.
Results: Among the 44 patients enrolled in this study, 28 BRAF-wildtype, 9 BRAF-mutated, and 7 NRAS-mutated patients were identified. OS analysis showed a significant difference between wildtype and BRAF- or NRAS-mutated patients: 23.2 months vs 5.3 and 4.59, respectively, p = 0.017. The presence of brain metastases and BRAF or NRAS mutation were independent factors for mortality in multivariate analysis.
Conclusions: In case of failure to enroll patients in innovative clinical trials, second-line ipilimumab still represents an effective therapy in patients with metastatic wildtype melanoma and in the absence of brain metastases.
Keywords: BRAF; NRAS; immunotherapy; ipilimumab; melanoma.
Conflict of interest statement
FDG has been a speaker at BMS and Novartis conference. PM had a consultant/advisory role for BMS, ROCHE Genentech, MSD, Novartis, AMGEN, Merck Serono, Pierre Fabre, and INCYTE. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Other authors do not declare any conflicts of interest.
Figures



References
-
- van Not O.J., van den Eertwegh A.J.M., Jalving H., Bloem M., Haanen J.B., van Rijn R.S., Aarts M.J.B., van den Berkmortel F.W.P.J., Blank C.U., Boers-Sonderen M.J., et al. Long-Term Survival in Patients with Advanced Melanoma. JAMA Netw. Open. 2024;7:e2426641. doi: 10.1001/jamanetworkopen.2024.26641. - DOI - PMC - PubMed
-
- Robert C., Ribas A., Schachter J., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C.M., Lotem M., et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–1251. doi: 10.1016/S1470-2045(19)30388-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

