The Importance of Real-World Data in Evaluating the Safety of Biosimilars: A Descriptive Study of Clinical Practice in an Oncohematological Italian Population
- PMID: 39410038
- PMCID: PMC11476336
- DOI: 10.3390/cancers16193419
The Importance of Real-World Data in Evaluating the Safety of Biosimilars: A Descriptive Study of Clinical Practice in an Oncohematological Italian Population
Abstract
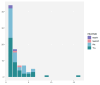
The clinical safety and efficacy of rituximab biosimilars compared to the reference rituximab (Mabthera) have been well established in randomized trials. However, concerns persist regarding the safety of changing from the reference product to biosimilars, and particularly between different biosimilars. This prospective multicenter observational study was conducted in 13 oncohematology units of eight Italian regions. The study included 800 patients with non-Hodgkin lymphoma (NHL) or chronic lymphocytic leukemia (CLL) who received rituximab between March 2018 and June 2022. To minimize survivorship bias, only newly diagnosed patients (i.e., those without prior rituximab treatment) were included in the analysis of adverse drug reactions (ADRs). Thus, this study focused on 505 incident cases (79.8% of the initial cohort) from 13 centers. A total of 3681 rituximab infusions were administered, and 16.8% of the patients experienced at least one ADR. These were observed most frequently during the first infusion (44 patients, 52%) and the second infusion (17 patients, 20%). The most frequent reactions were general disorders and administration site conditions (n. 50, 8% serious). These findings support the clinical safety of rituximab biosimilars and suggest that switching between biosimilars does not increase the risk of adverse events. This evidence may alleviate concerns about biosimilar use, potentially leading to broader acceptance and reduced healthcare costs.
Keywords: biosimilars; chronic lymphocytic leukemia (CLL); non-Hodgkin lymphoma (NHL); oncohematology; rituximab; safety.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- European Medicines Agency (EMA) Guideline on Similar Biological Medicinal Products Containing Biotechnology-Derived Proteins as Active Substance: Non-Clinical and Clinical Issues. 2013. [(accessed on 23 August 2024)]. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-si....
-
- World Health Organization (WHO) Guidelines on Evaluation of Biosimilars. [(accessed on 23 August 2024)]. Available online: https://www.who.int/publications/m/item/guidelines-on-evaluation-of-bios....
-
- US Food and Drug Administration (FDA) Biosimilar Guidance. [(accessed on 23 August 2024)]; Available online: https://www.fda.gov/vaccines-blood-biologics/general-biologics-guidances....
LinkOut - more resources
Full Text Sources
Research Materials

