Coal to Clean: Comparing Advanced Electrodes for Desulfurization and Copper Recovery
- PMID: 39410361
- PMCID: PMC11478058
- DOI: 10.3390/ma17194790
Coal to Clean: Comparing Advanced Electrodes for Desulfurization and Copper Recovery
Abstract
This study explores the electrochemical desulfurization of coal and the recovery of copper (Cu) using dimensionally stable anode (DSA) electrodes.
Background: The research addresses the need for effective sulfur removal from coal to reduce emissions.
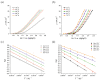
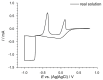
Methods: Electrochemical desulfurization was conducted using DSA and graphite electrodes, evaluating parameters like activation energy, desulfurization rate, and energy consumption. Cyclic voltammetry and linear sweep voltammetry were used to study the electrochemical properties.
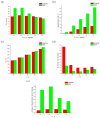
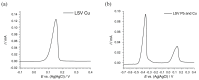
Results: The DSA electrode demonstrated superior performance with higher desulfurization rates, lower activation energy, and better response to temperature increases compared to the graphite electrode. Optimal desulfurization was achieved at 50 °C with the DSA electrode, balancing efficiency and energy consumption. Copper recovery from the solution post-desulfurization was effective, with an 86.34% recovery rate at -0.15 V vs. (Ag|AgCl). The energy consumption for the Cu recovery was calculated to be 10.56 J, and the total cost for recovering 1 ton of Cu was approximately 781.20 €.
Conclusions: The study highlights the advantages of DSA electrodes for efficient sulfur removal and metal recovery, promoting cleaner energy production and environmental sustainability. Future research should focus on optimizing electrochemical conditions and scaling up the process for industrial applications.
Keywords: coal treatment; copper recovery; dimensionally stable anode (DSA); electrochemical desulfurization; energy efficiency; graphite electrode; sulfur removal.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Luo H., Li T., Wang F., Cui K. Study on the Effect of Ash in Coal Liquefaction Residue on the Preparation of High-Density Carbon Materials by Induced Polycondensation. Energy Sources Part A Recovery Util. Environ. Eff. 2022;44:340–352. doi: 10.1080/15567036.2022.2046214. - DOI
-
- Zhang Y., Yu Q., Wang H., Zou M. Study on the Performance of Petroleum Coke after Electrolytic Desulfurization in NaBr-CH3COOH System. Energy Sources Part A Recovery Util. Environ. Eff. 2021:1–16. doi: 10.1080/15567036.2021.1985189. - DOI
-
- Pantovic-Spajic K., Markovic B., Pavlovic M., Sokic M., Zildzovic S., Djordjevic N., Stojanovic K. Deashing and Desulfurization of Subbituminous Coal from the East Field (Bogovina Basin, Serbia)—Insights from Chemical Leaching. J. Serbian Chem. Soc. 2021;86:1113–1126. doi: 10.2298/JSC210719061P. - DOI
-
- Shan Y., Guan D., Meng J., Liu Z., Schroeder H., Liu J., Mi Z. Rapid Growth of Petroleum Coke Consumption and Its Related Emissions in China. Appl. Energy. 2018;226:494–502. doi: 10.1016/j.apenergy.2018.06.019. - DOI
LinkOut - more resources
Full Text Sources

