Impact of the 2023 ACR/EULAR Classification Criteria in Women with Primary Antiphospholipid Syndrome during Pregnancy
- PMID: 39410566
- PMCID: PMC11475906
- DOI: 10.3390/diagnostics14192162
Impact of the 2023 ACR/EULAR Classification Criteria in Women with Primary Antiphospholipid Syndrome during Pregnancy
Abstract
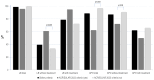
Background/Objectives: ACR/EULAR has recently developed new classification criteria for antiphospholipid syndrome (APS). The present study aims to analyze the impact of these new 2023 ACR/EULAR classification criteria in a cohort of pregnant women with primary APS. Methods: Retrospective cohort study of 93 consecutive pregnant women attending the Autoimmune Diseases Pregnancy Clinic, a multidisciplinary unit of a tertiary care teaching hospital, between 2005 and 2023. All of them fulfilled the Sydney classification criteria for APS. Women diagnosed with rheumatic autoimmune diseases other than APS were excluded. Results: Twenty-four out of ninety-three patients (25.8%) met the 2023 ACR/EULAR criteria for APS. Patients who met the new classification criteria were very similar to those who did not, except for being younger (p < 0.001), and had a lower number of clinical pregnancies (p = 0.004). The obstetric domain was clearly underrepresented in women who fulfilled the 2023 ACR/EULAR criteria (p < 0.001). Patients meeting the new classification criteria were primarily characterized by preterm births before 34 weeks due to severe placentation disorders (p = 0.004). Women with early and late fetal loss were significantly underrepresented (p < 0.0001 and 0.03, respectively). Nearly half of these patients had thrombocytopenia (p < 0.001). Serologically, these patients showed a higher frequency of persistent lupus anticoagulant (p = 0.02) and a lower frequency of IgM isotype antiphospholipid antibodies (p = 0.05). Conclusions: Almost three-quarters of the patients included in the study did not meet the 2023 ACR/EULAR criteria. Most patients who could not be classified according to these new classification criteria were those with early and/or late fetal deaths, as well as patients carrying only IgM aCL/AB2GPI antibodies. The high specificity of the 2023 ACR/EULAR criteria, restricted to severe placentation disorders, may leave the majority of patients with obstetric APS out of the new classification criteria.
Keywords: antiphospholipid antibodies; antiphospholipid syndrome; classification criteria; fetal loss; obstetric morbidity; pregnancy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Miyakis S., Lockshin M.D., Atsumi T., Branch D.W., Brey R.L., Cervera R., Derkesen R.H.W.M., De Groot P.G., Koike T., Meroni P.L., et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS) J. Thromb. Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. - DOI - PubMed
-
- Barbhaiya M., Zuily S., Ahmadzadeh Y., Amigo M.C., Avcin T., Bertolaccini M.L., Branch D.W., de Jesus G., Devreese K.M.J., Frances C., et al. Development of a New International Antiphospholipid Syndrome Classification Criteria Phase I/II Report: Generation and Reduction of Candidate Criteria. Arthritis Care Res. 2021;73:1490–1501. doi: 10.1002/acr.24520. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

