Internal Quality Controls in the Medical Laboratory: A Narrative Review of the Basic Principles of an Appropriate Quality Control Plan
- PMID: 39410627
- PMCID: PMC11475633
- DOI: 10.3390/diagnostics14192223
Internal Quality Controls in the Medical Laboratory: A Narrative Review of the Basic Principles of an Appropriate Quality Control Plan
Abstract
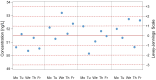
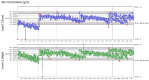
To ensure the quality of their analyses, medical laboratories carry out internal quality control (IQC) on a daily basis. IQC involves control samples with known target values for all parameters used by a laboratory in clinical practice. The use of IQC enables the laboratory to monitor the accuracy and precision of laboratory results. The use of appropriate IQC strategies has been accepted in medical laboratories for decades, and IQC has been included in international recommendations and guidelines. The term "IQC strategy" (also termed a quality control plan) refers to the types of IQC materials to be measured, the frequency of IQC events, the number of concentration levels in each IQC event, and the IQC rules to be used. A scientifically sound IQC strategy must follow two principles, namely, (1) statistical follow-up on the IQC results generated in the laboratory by means of Levey-Jennings control charts and Westgard rules (i.e., quality control by means of statistical procedures) and (2) the determination of limits on the basis of medical considerations and the definition of analytical goals (quality control on the basis of medical relevance). In this narrative review, we describe the fundamental principles of an adequate IQC strategy for laboratorians and nonlaboratorians.
Keywords: bias (MeSH ID: D015982); diagnostic errors (MeSH ID: D003951); laboratories, clinical (MeSH ID: D000090464); quality control (MeSH ID: D011786); risk assessment (MeSH ID: D018570).
Conflict of interest statement
L.G., A.H. and T.M. declare that they have no competing interests.
Figures
References
-
- Hallworth M.J., Epner P.L., Ebert C., Fantz C.R., Faye S.A., Higgins T.N., Kilpatrick E.S., Li W., Rana S.V., Vanstapel F., et al. Current evidence and future perspectives on the effective practice of patient-centered laboratory medicine. Clin. Chem. 2015;61:589–599. doi: 10.1373/clinchem.2014.232629. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

