Are pragmatism and ethical protections in clinical trials a zero-sum game?
- PMID: 39410779
- PMCID: PMC11809111
- DOI: 10.1177/17407745241284798
Are pragmatism and ethical protections in clinical trials a zero-sum game?
Abstract
Background: Randomized controlled trials with pragmatic intent aim to generate evidence that directly informs clinical decisions. Some have argued that the ethical protection of informed consent can be in tension with the goals of pragmatism. But the impact of other ethical protections on trial pragmatism has yet to be explored.
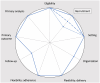
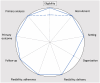
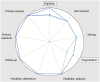
Purpose: In this article, we analyze the relationship between additional ethical protections for vulnerable participants and the degree of pragmatism within the PRagmatic Explanatory Continuum Indicator Summary-2 (PRECIS-2) domains of trial design.
Methods: We analyze three example trials with pragmatic intent that include vulnerable participants.
Conclusion: The relationship between ethical protections and trial pragmatism is complex. In some cases, additional ethical protections for vulnerable participants can promote the pragmatism of some of the PRECIS-2 domains of trial design. When designing trials with pragmatic intent, researchers ought to look for opportunities wherein ethical protections enhance the degree of pragmatism.
Keywords: Pragmatic trials; critical care; geriatrics; psychiatry; research ethics; vulnerability.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: C.W. receives consulting income from Cardialen, Eli Lilly & Company. H.P.N. is a co-author of the published PRiVII Trial protocol and is a member of the research team coordinating the trial. M.T. has no conflicts of interest to declare.
Figures
Similar articles
-
The ethical challenges raised in the design and conduct of pragmatic trials: an interview study with key stakeholders.Trials. 2019 Dec 23;20(1):765. doi: 10.1186/s13063-019-3899-x. Trials. 2019. PMID: 31870433 Free PMC article.
-
Ethical Issues in Pragmatic Cluster-Randomized Trials in Dialysis Facilities.Am J Kidney Dis. 2019 Nov;74(5):659-666. doi: 10.1053/j.ajkd.2019.04.019. Epub 2019 Jun 19. Am J Kidney Dis. 2019. PMID: 31227227 Free PMC article. Review.
-
Cluster over individual randomization: are study design choices appropriately justified? Review of a random sample of trials.Clin Trials. 2020 Jun;17(3):253-263. doi: 10.1177/1740774519896799. Epub 2020 May 5. Clin Trials. 2020. PMID: 32367741 Review.
-
A limited number of medicines pragmatic trials had potential for waived informed consent following the 2016 CIOMS ethical guidelines.J Clin Epidemiol. 2019 Oct;114:60-71. doi: 10.1016/j.jclinepi.2019.06.007. Epub 2019 Jun 15. J Clin Epidemiol. 2019. PMID: 31212001
-
Ethical and Regulatory Issues for Embedded Pragmatic Trials Involving People Living with Dementia.J Am Geriatr Soc. 2020 Jul;68 Suppl 2(Suppl 2):S37-S42. doi: 10.1111/jgs.16620. J Am Geriatr Soc. 2020. PMID: 32589273 Free PMC article.
References
-
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutic trials. J Chron Dis 1967; 20: 637–648. - PubMed
-
- Loudon K, Trweek S, Sullivan F, et al.. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015; 350: h2147. - PubMed
-
- Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016; 375: 454–463. - PubMed
-
- Pletcher MJ, Lo B, Grady D. Informed consent in randomized quality improvement trials: a critical barrier for learning health systems. JAMA Intern Med 2014; 174(5): 668–670. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

