Manifold-Fused Porphyrin-Nanographene Conjugates
- PMID: 39410809
- PMCID: PMC11632405
- DOI: 10.1002/chem.202403250
Manifold-Fused Porphyrin-Nanographene Conjugates
Abstract
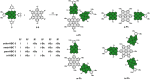
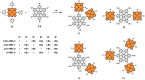
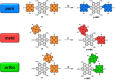
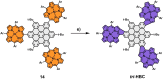
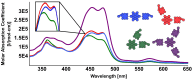
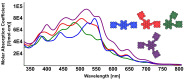
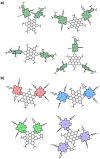
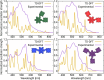
A library of novel π-extended porphyrin-hexabenzocoronene (HBC) architectures is presented. Two distinct synthetic pathways were utilized to obtain either phenyl- or HBC-fused compounds. Absorption experiments reveal the species' exciting photophysical and optoelectronic properties. Depending on the degree of π-extension, the number of porphyrins, and their relative position, a decisive change in shape, panchromatic broadening, and red-shifting of the absorption curves is observed. Theoretical studies give more profound insight into the molecule's electronic structures, showing vast decreases in HOMO-LUMO energy gaps.
Keywords: Fusion reaction; Porphyrinoids; Post-functionalization; Scholl oxidation; π-Extension.
© 2024 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Grumbling E., Horowitz M., Quantum Computing, National Academies Press, Washington, D. C. 2019.
-
- Gyongyosi L., Imre S., Comput. Sci. Rev. 2019, 31, 51.
-
- Shalf J., Philos. Transact. A, Math. Phys. Eng. Sci. 2020, 378, 20190061. - PubMed
-
- Razavi B., Fundamentals of Microelectronics. with Robotics and Bioengineering applications, Wiley, Hoboken: 2021.
-
- Schaller R. R., IEEE Spectrum 1997, 34, 52.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

