Hemoglobin α-derived peptides VD-hemopressin (α) and RVD-hemopressin (α) are involved in electroacupuncture inhibition of chronic pain
- PMID: 39411061
- PMCID: PMC11473328
- DOI: 10.3389/fphar.2024.1439448
Hemoglobin α-derived peptides VD-hemopressin (α) and RVD-hemopressin (α) are involved in electroacupuncture inhibition of chronic pain
Abstract
Introduction: Knee osteoarthritis (KOA) is a chronic degenerative bone metabolic disease that primarily affects older adults, leading to chronic pain and disability that affect patients' daily activities. Electroacupuncture (EA) is a commonly used method for the treatment of chronic pain in clinical practice. Previous studies indicate that the endocannabinoid system is involved in EA analgesia, but whether endocannabinopeptide VD-hemopressin (α) and RVD-hemopressin (α) derived from hemoglobin chains are involved in EA analgesia is unclear.
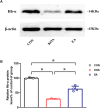
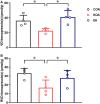
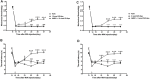
Methods: RNA-seq technology was used to screen which genes involved in EA analgesia. The expression of hemoglobin α chain and 26S proteasome were determined by Western blotting. The level of VD-hemopressin (α) and RVD-hemopressin (α) were measured by UPLC-MS/MS. Microinjection VD-Hemopressin (α), RVD-Hemopressin (α) and 26S proteasome inhibitor MG-132 into vlPAG, then observe mechanical and thermal pain thresholds.
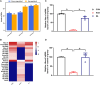
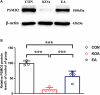
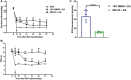
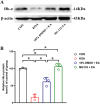
Results: Therefore, we used RNA-seq to obtain differentially expressed genes Hba-a1 and Hba-a2 involved in EA analgesia in the periaqueductal gray (PAG), which were translated into the hemoglobin α chain. EA significantly increased the expression of the hemoglobin α chain and the level of hemopressin (α) and RVD-hemopressin (α). Microinjection of VD-hemopressin (α) and RVD-hemopressin (α) into the ventrolateral periaqueductal gray (vlPAG) mimicked the analgesic effect of EA, while CB1 receptor antagonist AM251 reversed this effect. EA significantly increased the expression of 26S proteasome in KOA mice. Microinjection of 26S proteasome inhibitor MG132 before EA prevented both the anti-allodynic effect and upregulation of the concentration of RVD-hemopressin (α) by EA treatment and upregulated the expression of the hemoglobin α chain.
Discussion: Our data suggest that EA upregulated the concentration of VD-hemopressin (α) and RVD-hemopressin (α) through enhancement of the hemoglobin α chain degradation by 26S proteasome in the PAG, then activated the CB1 receptor, thereby exerting inhibition of chronic pain in a mouse model of KOA. These results provide new insights into the EA analgesic mechanisms and reveal possible targets for EA treatment of chronic pain.
Keywords: 26S proteasome; Chronic pain; Electroacupuncture analgesia; Knee osteoarthritis (KOA); RVD-hemopressin (α); VD-hemopressin (α).
Copyright © 2024 Yuan, Guo, Yi, Hou, Zhao, Wang, Jia, Baba, Li and Huo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bauer M., Chicca A., Tamborrini M., Eisen D., Lerner R., Lutz B., et al. (2012). Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 287 (44), 36944–36967. 10.1074/jbc.M112.382481 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

