Reprogramming with Atoh1, Gfi1, and Pou4f3 promotes hair cell regeneration in the adult organ of Corti
- PMID: 39411090
- PMCID: PMC11477985
- DOI: 10.1093/pnasnexus/pgae445
Reprogramming with Atoh1, Gfi1, and Pou4f3 promotes hair cell regeneration in the adult organ of Corti
Abstract
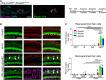
Cochlear hair cells can be killed by loud noises, ototoxic drugs, and natural aging. Once lost, mammalian hair cells do not naturally regenerate, leading to permanent hearing loss. Since the mammalian cochlea lacks any intrinsic ability to regenerate, genetic reprogramming of cochlear supporting cells that lie adjacent to hair cells is a potential option for hearing restoration therapies. We targeted cochlear supporting cells with three hair cell transcription factors: Atoh1, or Atoh1 + Gfi1, or Atoh1 + Gfi1 + Pou4f3 and found that 1- and 2-factor reprogramming is not sufficient to reprogram adult supporting cells into hair cells. However, activation of all three hair cell transcription factors reprogrammed some adult supporting cells into hair cell-like cells. We found that killing endogenous hair cells significantly improved the ability of supporting cells to be reprogrammed and regenerated numerous hair cell-like cells throughout the length of the cochlea. These regenerated hair cell-like cells expressed myosin VIIa and parvalbumin, as well as the mature outer hair cell protein prestin, were innervated, expressed proteins associated with ribbon synapses, and formed rudimentary stereociliary bundles. Finally, we demonstrate that supporting cells remained responsive to transcription factor reprogramming for at least 6 weeks after hair cell damage, suggesting that hair cell reprogramming may be effective in the chronically deafened cochlea.
Keywords: hair cells; inner ear; reprogramming.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures



References
-
- Azizi MH. 2010. Occupational noise-induced hearing loss. Int J Occup Environ Med. 1:116–123. - PubMed
-
- Mifsud M, Boyev KP. 2015. Ototoxicity. In: Hamilton and Hardy's Industrial toxicology. 6th ed. Vol 72. John Wiley & Sons, Inc. p. 1241–1246.
-
- Henley CM, Rybak LP. 1995. Ototoxicity in developing mammals. Brain Res Rev. 20:68–90. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

