Inhibition of ATM or ATR in combination with hypo-fractionated radiotherapy leads to a different immunophenotype on transcript and protein level in HNSCC
- PMID: 39411143
- PMCID: PMC11473424
- DOI: 10.3389/fonc.2024.1460150
Inhibition of ATM or ATR in combination with hypo-fractionated radiotherapy leads to a different immunophenotype on transcript and protein level in HNSCC
Erratum in
-
Corrigendum: Inhibition of ATM or ATR in combination with hypo-fractionated radiotherapy leads to a different immunophenotype on transcript and protein level in HNSCC.Front Oncol. 2025 Apr 23;15:1607882. doi: 10.3389/fonc.2025.1607882. eCollection 2025. Front Oncol. 2025. PMID: 40337061 Free PMC article.
Abstract
Background: The treatment of head and neck tumors remains a challenge due to their reduced radiosensitivity. Small molecule kinase inhibitors (smKI) that inhibit the DNA damage response, may increase the radiosensitivity of tumor cells. However, little is known about how the immunophenotype of the tumor cells is modulated thereby. Therefore, we investigated whether the combination of ATM or ATR inhibitors with hypo-fractionated radiotherapy (RT) has a different impact on the expression of immune checkpoint markers (extrinsic), the release of cytokines or the transcriptome (intrinsic) of head and neck squamous cell carcinoma (HNSCC) cells.
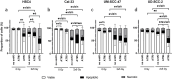
Methods: The toxic and immunogenic effects of the smKI AZD0156 (ATMi) and VE-822 (ATRi) in combination with a hypo-fractionated scheme of 2x5Gy RT on HPV-negative (HSC4, Cal-33) and HPV-positive (UM-SCC-47, UD-SCC-2) HNSCC cell lines were analyzed as follows: cell death (necrosis, apoptosis; detected by AnxV/PI), expression of immunostimulatory (ICOS-L, OX40-L, TNFSFR9, CD70) and immunosuppressive (PD-L1, PD-L2, HVEM) checkpoint marker using flow cytometry; the release of cytokines using multiplex ELISA and the gene expression of Cal-33 on mRNA level 48 h post-RT.
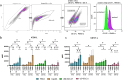
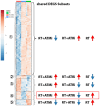
Results: Cell death was mainly induced by the combination of RT with both inhibitors, but stronger with ATRi. Further, the immune phenotype of cancer cells, not dying from combination therapy itself, is altered predominantly by RT+ATRi in an immune-stimulatory manner by the up-regulation of ICOS-L. However, the analysis of secreted cytokines after treatment of HNSCC cell lines revealed an ambivalent influence of both inhibitors, as we observed the intensified secretion of IL-6 and IL-8 after RT+ATRi. These findings were confirmed by RNAseq analysis and further the stronger immune-suppressive character of RT+ATMi was enlightened. We detected the down-regulation of a central protein of cytoplasmatic sensing pathways of nucleic acids, RIG-1, and found one immune-suppressive target, EDIL3, strongly up-regulated by RT+ATMi.
Conclusion: Independent of a restrictive toxicity, the combination of RT + either ATMi or ATRi leads to comprehensive and immune-modulating alterations in HNSCC. This includes pro-inflammatory signaling induced by RT + ATRi but also anti-inflammatory signals. These findings were confirmed by RNAseq analysis, which further highlighted the immune-suppressive nature of RT + ATMi.
Keywords: ATM inhibition; ATR inhibition; DNA damage repair; HNSCC; immunomodulation; kinase inhibitors.
Copyright © 2024 Meidenbauer, Wachter, Schulz, Mostafa, Zülch, Frey, Fietkau, Gaipl and Jost.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

