Controlled infection with cryopreserved human hookworm induces CTLA-4 expression on Tregs and upregulates tryptophan metabolism
- PMID: 39411786
- PMCID: PMC11485773
- DOI: 10.1080/19490976.2024.2416517
Controlled infection with cryopreserved human hookworm induces CTLA-4 expression on Tregs and upregulates tryptophan metabolism
Abstract
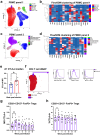
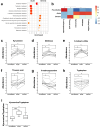
Infecting humans with controlled doses of helminths, such as human hookworm (termed hookworm therapy), is proposed to prevent or treat various intestinal and extraintestinal diseases. However, full-scale clinical trials examining hookworm therapy are limited by the inability to scale-up the production of hookworm larvae to infect sufficient numbers of patients. With the aim of overcoming this challenge, this study infected four healthy individuals with hookworm larvae that had been reanimated from cryopreserved eggs to examine their viability and immunogenicity. We demonstrate that reanimated cryopreserved hookworm larvae establish a viable hookworm infection and elicit a similar immune response to larvae cultured from fresh stool. Furthermore, a refined understanding of the therapeutic mechanisms of hookworm is imperative to determine which diseases to target with hookworm therapy. To investigate potential therapeutic mechanisms, this study assessed changes in the immune cells, microbiome, and plasma metabolome in the four healthy individuals infected with cryopreserved hookworm larvae and another nine individuals infected with larvae cultured from freshly obtained stool. We identified potential immunoregulatory mechanisms by which hookworm may provide a beneficial effect on its host, including increased expression of CTLA-4 on regulatory T cells (Tregs) and upregulation of tryptophan metabolism. Furthermore, we found that a participant's baseline microbiome predicted the severity of symptoms and intestinal inflammation experienced during a controlled hookworm infection. In summary, our findings demonstrate the feasibility of full-scale clinical trials examining hookworm therapy by minimizing the reliance on human donors and optimizing the culturing process, thereby enabling viable hookworm larvae to be mass-produced and enabling on-demand inoculation of patients. Furthermore, this study provides insights into the complex interactions between helminths and their host, which could inform the development of novel therapeutic strategies.
Keywords: Hookworm; clinical trial; controlled infection; cryopreservation; eosinophilia; helminths; immune regulation; microbiome.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Mules TC, Inns S, Le Gros G.. Helminths’ therapeutic potential to treat intestinal barrier dysfunction. Allergy. 2023 Jul 14;all.15812. - PubMed
-
- Feary J, Venn A, Brown A, Hooi D, Falcone FH, Mortimer K, Pritchard DI, Britton J. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin Exp Allergy. 2009. Jul. 39(7):1060–17. doi:10.1111/j.1365-2222.2009.03187.x. - DOI - PMC - PubMed
-
- Tanasescu R, Tench CR, Constantinescu CS, Telford G, Singh S, Frakich N, Onion D, Auer DP, Gran B, Evangelou N, et al. Hookworm treatment for relapsing multiple sclerosis: a randomized double-blinded placebo-controlled trial. JAMA Neurol. [2020 Sep 1]. 77(9):1089. doi:10.1001/jamaneurol.2020.1118. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
