HaloTag-Modified, Ferrocene Labeled Self-Assembled Monolayers for Protein Sensing
- PMID: 39411850
- PMCID: PMC12284956
- DOI: 10.1021/acs.langmuir.4c02941
HaloTag-Modified, Ferrocene Labeled Self-Assembled Monolayers for Protein Sensing
Abstract
Ferrocene (Fc)-based disulfide molecules of various lengths with amino acid scaffolds and alkane or oligo(phenylene-ethynylene) (OPE) bridges are used in a mixed SAM with a di-(ethylene oxide) terminal mercaptoundecanol diluent (PEG2). The relative height of the Fc redox reporter in the SAM is compared to determine if there are protective effects like antifouling and specific detection. The HaloTag-binding motif is used as a proof-of-concept to investigate the electrochemical response to the HaloTag protein due to its known covalent and fast linkage. When the Fc-SAMs are exposed to the HaloTag protein, there are an antifouling nature and more specific detection for the engulfed Fc-based molecules (C6tBu/Halo). The further out the Fc is from the SAM layer, the more nonspecific adsorption is detected. The double layer capacitance (CDL) has the smallest change for the C6tBu control (ΔCDL = -0.1 μF cm-2) showing antifouling properties and produces a large change (ΔCDL = 0.9 μF cm-2) as well as a shift in oxidation potential when the active C6Halo is exposed to the HaloTag protein (ΔE1/2 = 50 ± 10 mV). The remaining Fc molecules are partially in or outside the PEG2 layer, allowing more ion penetration/mobility even when the HaloTag protein is bound. Generally, a more disordered environment was observed for the Fc-based molecules when adding the HaloTag ligand, which is evident from a larger Efwhm and higher CDL. Desorption of the SAMs with sodium iodide (NaI) showed retention of the HaloTag protein bound with the corresponding ligand, whereas negative controls did not. Self-assembled monolayers for MALDI mass spectrometry (SAMDI-MS) were used as an orthogonal detection technique to show the qualitative binding of the HaloTag protein to the electrode. Together, these results provide insight into the antifouling and detection methods of engulfing the redox molecules in the SAM diluent.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

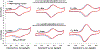
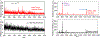
References
-
- Love JC; Estroff LA; Kriebel JK; Nuzzo RG; Whitesides GM Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105 (4), 1103–1169. - PubMed
-
- Creager SE; Rowe GK Redox Properties of Ferrocenalkane Thiols Coadsorbed with Linear Normal-Alkanethiols on Polycrystal-line Bulk Gold Electrodes. Anal. Chim. Acta 1991, 246 (1), 233–239.
-
- Sumner JJ; Creager SE Redox kinetics in monolayers on electrodes: Electron transfer is sluggish for ferrocene groups buried within the monolayer interior. J. Phys. Chem. B 2001, 105 (37), 8739–8745.
-
- Bain CD; Evall J; Whitesides GM Formation of Monolayers by The Coadsorption of Thiols on Gold - Variation in the Head Group, Tail Group, and Solvent. J. Am. Chem. Soc. 1989, 111 (18), 7155–7164.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

