An immune signature of postoperative cognitive decline: a prospective cohort study
- PMID: 39411891
- PMCID: PMC11634152
- DOI: 10.1097/JS9.0000000000002118
An immune signature of postoperative cognitive decline: a prospective cohort study
Abstract
Background: Postoperative cognitive decline (POCD) is the predominant complication affecting patients over 60 years old following major surgery, yet its prediction and prevention remain challenging. Understanding the biological processes underlying the pathogenesis of POCD is essential for identifying mechanistic biomarkers to advance diagnostics and therapeutics. This study aimed to provide a comprehensive analysis of immune cell trajectories differentiating patients with and without POCD and to derive a predictive score enabling the identification of high-risk patients during the preoperative period.
Material and methods: Twenty-six patients aged 60 years old and older undergoing elective major orthopedic surgery were enrolled in a prospective longitudinal study, and the occurrence of POCD was assessed 7 days after surgery. Serial samples collected before surgery, and 1, 7, and 90 days after surgery were analyzed using a combined single-cell mass cytometry and plasma proteomic approach. Unsupervised clustering of the high-dimensional mass cytometry data was employed to characterize time-dependent trajectories of all major innate and adaptive immune cell frequencies and signaling responses. Sparse machine learning coupled with data-driven feature selection was applied to the presurgery immunological dataset to classify patients at risk for POCD.
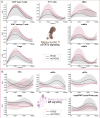
Results: The analysis identified cell-type and signaling-specific immune trajectories differentiating patients with and without POCD. The most prominent trajectory features revealed early exacerbation of JAK/STAT and dampening of inhibitory κB and nuclear factor-κB immune signaling responses in patients with POCD. Further analyses integrating immunological and clinical data collected before surgery identified a preoperative predictive model comprising one plasma protein and 10 immune cell features that classified patients at risk for POCD with excellent accuracy (AUC=0.80, P =2.21e-02 U -test).
Conclusion: Immune system-wide monitoring of patients over 60 years old undergoing surgery unveiled a peripheral immune signature of POCD. A predictive model built on immunological data collected before surgery demonstrated greater accuracy in predicting POCD compared to known clinical preoperative risk factors, offering a concise list of biomarker candidates to personalize perioperative management.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
J.H., B.G., and F.V. are listed as inventors on a patent application (PCT/US2023/074903). J.H., B.G., D.G., and F.V. are advisory board members, G.B. is employed, and E.G. is a consultant at SurgeCare SAS. The remaining authors declare no competing interests.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures



Update of
-
An immune signature of postoperative cognitive decline in elderly patients.bioRxiv [Preprint]. 2024 Mar 5:2024.03.02.582845. doi: 10.1101/2024.03.02.582845. bioRxiv. 2024. Update in: Int J Surg. 2024 Dec 01;110(12):7749-7762. doi: 10.1097/JS9.0000000000002118. PMID: 38496400 Free PMC article. Updated. Preprint.
References
-
- Meara JG, Leather AJM, Hagander L, et al. . Global surgery 2030: evidence and solutions for achieving health, welfare, and economic development. The Lancet 2015;386:569–624. - PubMed
-
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455–1460. - PubMed
-
- Monk TG, Weldon BC, Garvan CW, et al. . Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008;108:18–30. - PubMed
-
- Moller JT, Cluitmans P, Rasmussen LS, et al. . Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. The Lancet 1998;351:857–861. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

