Serum Glial Fibrillary Acidic Protein and Neurofilament Light Chain Levels Reflect Different Mechanisms of Disease Progression under B-Cell Depleting Treatment in Multiple Sclerosis
- PMID: 39411917
- PMCID: PMC11683165
- DOI: 10.1002/ana.27096
Serum Glial Fibrillary Acidic Protein and Neurofilament Light Chain Levels Reflect Different Mechanisms of Disease Progression under B-Cell Depleting Treatment in Multiple Sclerosis
Abstract
Objective: To investigate the longitudinal dynamics of serum glial fibrillary acidic protein (sGFAP) and serum neurofilament light chain (sNfL) levels in people with multiple sclerosis (pwMS) under B-cell depleting therapy (BCDT) and their capacity to prognosticate future progression independent of relapse activity (PIRA) events.
Methods: A total of 362 pwMS (1,480 samples) starting BCDT in the Swiss Multiple Sclerosis (MS) Cohort were included. sGFAP levels in 2,861 control persons (4,943 samples) provided normative data to calculate adjusted Z scores.
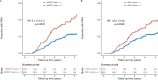
Results: Elevated sGFAP levels (Z score >1) at 1 year were associated with a higher hazard for PIRA (hazard ratio [HR]: 1.80 [95% CI: 1.17-2.78]; p = 0.0079) than elevated sNfL levels (HR, 1.45 [0.95-2.24], p = 0.0886) in a combined model. Independent of PIRA events, sGFAP levels longitudinally increased by 0.49 Z score units per 10 years follow-up (estimate, 0.49 [0.29, 0.69], p < 0.0001). In patients experiencing PIRA, sGFAP Z scores were 0.52 Z score units higher versus stable patients (0.52 [0.22, 0.83], p = 0.0009). Different sNfL Z score trajectories were found in pwMS with versus without PIRA (interaction p = 0.0028), with an average decrease of 0.92 Z score units per 10 years observed without PIRA (-0.92 [-1.23, -0.60], p < 0.0001), whereas levels in patients with PIRA remained high.
Interpretation: Elevated sGFAP and lack of drop in sNfL after BCDT start are associated with increased risk of future PIRA. These findings provide a rationale for combined monitoring of sNfL and sGFAP in pwMS starting BCDT to predict the risk of PIRA, and to use sGFAP as an outcome in clinical trials aiming to impact on MS progressive disease biology. ANN NEUROL 2024.
© 2024 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures