Future investigative directions for novel therapeutic targets in head and neck cancer
- PMID: 39412140
- PMCID: PMC11514385
- DOI: 10.1080/14737140.2024.2417038
Future investigative directions for novel therapeutic targets in head and neck cancer
Abstract
Areas covered: Here we describe novel agents, their mechanism(s) of action, preclinical results, and ongoing clinical trials in HNSCC.
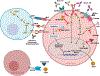
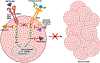
Expert opinion: Established therapeutic targets in HNSCC include EGFR (cetuximab) and PD-1 (pembrolizumab and nivolumab). Despite the detection of many other possible targets in HNSCC cell lines and patient tumors, no other therapies have successfully advanced to date. Identification of predictive biomarkers may guide the use of targeted agents and combination therapies. Clinical trials supported by strong preclinical data in relevant models are more likely to advance treatment options.
Keywords: HNSCC; PI3K inhibitors; breakthough designation; immunotherapy; radiation therapy.
Conflict of interest statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
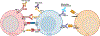
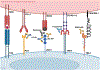
References
-
- Lippard SJ. New chemistry of an old molecule: cis-[Pt(NH3)2Cl2]. Science. 1982;218(4577):1075–82 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
