Methamphetamine-related working memory difficulties underpinned by reduced frontoparietal responses
- PMID: 39412242
- PMCID: PMC11480971
- DOI: 10.1111/adb.13444
Methamphetamine-related working memory difficulties underpinned by reduced frontoparietal responses
Abstract
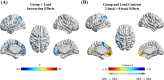
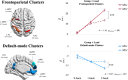
Working memory difficulties are common, debilitating, and may pose barriers to recovery for people who use methamphetamine. Yet, little is known regarding the neural dysfunctions accompanying these difficulties. Here, we acquired cross-sectional, functional magnetic resonance imaging while people with problematic methamphetamine-use experience (MA+, n = 65) and people without methamphetamine-use experience (MA-, n = 44) performed a parametric n-back task (0-back through 2-back). Performance on tasks administered outside of the scanner, together with n-back performance, afforded to determine a latent dimension of participants' working memory ability. Behavioural results indicated that MA+ participants exhibited lower scores on this dimension compared to MA- participants (d = -1.39, p < .001). Whole-brain imaging results also revealed that MA+ participants exhibited alterations in load-induced responses predominantly in frontoparietal and default-mode areas. Specifically, while the MA- group exhibited monotonic activation increases within frontoparietal areas and monotonic decreases within default-mode areas from 0-back to 2-back, MA+ participants showed a relative attenuation of these load-induced activation patterns (d = -1.55, p < .001). Moreover, increased activations in frontoparietal areas from 0- to 2-back were related to greater working memory ability among MA+ participants (r = .560, p = .004). No such effects were observed for default-mode areas. In sum, reductions in working memory ability were observed alongside load-induced dysfunctions in frontoparietal and default-mode areas for people with problematic methamphetamine-use experience. Among them, load-induced activations within frontoparietal areas were found to have a strong and specific relationship to individual differences in working memory ability, indicating a putative neural signature of the working memory difficulties associated with chronic methamphetamine use.
Keywords: default mode; fMRI; frontoparietal; methamphetamine; working memory.
© 2024 The Author(s). Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
The authors do not declare any known conflicts of interest related to this work.
Figures



References
-
- Alizadehgoradel J, Nejati V, Sadeghi Movahed F, et al. Repeated stimulation of the dorsolateral‐prefrontal cortex improves executive dysfunctions and craving in drug addiction: a randomized, double‐blind, parallel‐group study. Brain Stimul. 2020;13(3):582‐593. doi:10.1016/j.brs.2019.12.028 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

