Combination of FDG PET/CT radiomics and clinical parameters for outcome prediction in patients with non-Hodgkin's lymphoma
- PMID: 39412293
- PMCID: PMC11537470
- DOI: 10.1097/MNM.0000000000001895
Combination of FDG PET/CT radiomics and clinical parameters for outcome prediction in patients with non-Hodgkin's lymphoma
Abstract
Purpose: The purposes was to build model incorporating PET + computed tomography (CT) radiomics features from baseline PET/CT + clinical parameters to predict outcomes in patients with non-Hodgkin lymphomas.
Methods: Cohort of 138 patients with complete clinical parameters and follow up times of 25.3 months recorded. Textural analysis of PET and manual correlating contouring in CT images analyzed using LIFE X software. Defined outcomes were overall survival (OS), disease free-survival, radiotherapy, and unfavorable response (defined as disease progression) assessed by end of therapy PET/CT or contrast CT. Univariable and multivariable analysis performed to assess association between PET, CT, and clinical.
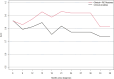
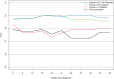
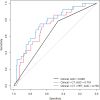
Results: Male ( P = 0.030), abnormal lymphocytes ( P = 0.030), lower value of PET entropy ( P = 0.030), higher value of SHAPE sphericity ( P = 0.002) were significantly associated with worse OS. Advanced stage (III or IV, P = 0.013), abnormal lymphocytes ( P = 0.032), higher value of CT gray-level run length matrix (GLRLM) LRLGE mean ( P = 0.010), higher value of PET gray-level co-occurrence matrix energy angular second moment ( P < 0.001), and neighborhood gray-level different matrix (NGLDM) busyness mean ( P < 0.001) were significant predictors of shorter DFS. Abnormal lymphocyte ( P = 0.033), lower value of CT NGLDM coarseness ( P = 0.082), and higher value of PET GLRLM gray-level nonuniformity zone mean ( P = 0.040) were significant predictors of unfavorable response to chemotherapy. Area under the curve for the three models (clinical alone, clinical + PET parameters, and clinical + PET + CT parameters) were 0.626, 0.716, and 0.759, respectively.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019; 94:604–616. - PubMed
-
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. . Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010; 116:2040–2045. - PMC - PubMed
-
- Gleeson M, Counsell N, Cunningham D, Lawrie A, Clifton-Hadley L, Hawkes E, et al. . Prognostic indices in diffuse large B-cell lymphoma in the rituximab era: an analysis of the UK National Cancer Research Institute R-CHOP 14 versus 21 phase 3 trial. Br J Haematol. 2021; 192:1015–1019. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

