Obesity-related drug transporter expression alterations in human liver and kidneys
- PMID: 39412582
- PMCID: PMC11582170
- DOI: 10.1007/s43440-024-00665-7
Obesity-related drug transporter expression alterations in human liver and kidneys
Abstract
Background: Pathophysiological changes associated with obesity might impact various drug pharmacokinetics (PK) parameters. The liver and kidneys are the primary organs involved in drug clearance, and the function of hepatic and renal transporters is critical to efficient drug elimination (or reabsorption). Considering the impact of an increased BMI on the drug's PK is crucial in directing dosing decisions. Given the critical role of transporters in drug biodisposition, this study investigated how overweight and obesity affect the gene expression of renal and hepatic drug transporters.
Methods: Human liver and kidney samples were collected post-mortem from 32 to 28 individuals, respectively, which were divided into the control group (lean subjects; 18.5 ≤ BMI < 25 kg/m2) and the study group (overweight/obese subjects; BMI ≥ 25 kg/m2). Real-time quantitative PCR was performed for the analysis of 84 drug transporters.
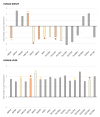
Results: Our results show significant changes in the expression of genes involved in human transporters, both renal and hepatic. In liver tissue, we found that ABCC4 was up-regulated in overweight/obese subjects. In kidney tissue, up-regulation was only observed for ABCC10, while the other differentially expressed genes were down-regulated: ABCA1, ABCC3, and SLC15A1.
Conclusions: The observed alterations may be reflected by the differences in drug PK between lean and obese populations. However, these findings need further evaluation through the proteomic and functional study of these transporters in this patient population.
Keywords: Drug transporters; Hepatic clearance; Obesity; Pharmacokinetics; Renal clearance.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: Necropsy specimens of liver and kidney were obtained from the National Disease Research Interchange (Philadelphia, PA, USA). The Rutgers Biomedical Health Sciences Institutional Review Board approved this study as exempt research (Pro2019001020).
Figures



References
-
- CDC. Obesity is a Common, Serious, and Costly Disease. Centers for Disease Control and Prevention 2022. https://www.cdc.gov/obesity/data/adult.html (accessed November 28, 2023).
-
- World Obesity Federation. World Obesity Atlas 2023. World Obesity Federation 2023. https://www.worldobesity.org/resources/resource-library/world-obesity-at... (accessed November 28, 2023).
-
- Lim Y, Boster J. Obesity and comorbid conditions. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. - PubMed
-
- Modesto ACF, Silveira EA, dos Santos Rodrigues AP, Lima DM, Provin MP, Amaral RG. Prevalence of Drug Interaction in severely obese individuals and Associated factors: baseline results from a clinical trial. Sci Pharm. 2020;88:48. 10.3390/scipharm88040048.
-
- Gouju J, Legeay S. Pharmacokinetics of obese adults: not only an increase in weight. Biomed Pharmacother. 2023;166:115281. 10.1016/j.biopha.2023.115281. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
