Coronary Revascularization Guided With Fractional Flow Reserve or Instantaneous Wave-Free Ratio: A 5-Year Follow-Up of the DEFINE FLAIR Randomized Clinical Trial
- PMID: 39412778
- PMCID: PMC11581635
- DOI: 10.1001/jamacardio.2024.3314
Coronary Revascularization Guided With Fractional Flow Reserve or Instantaneous Wave-Free Ratio: A 5-Year Follow-Up of the DEFINE FLAIR Randomized Clinical Trial
Erratum in
-
Errors in Author Affiliations and Open Access Updated.JAMA Cardiol. 2025 Jan 1;10(1):102. doi: 10.1001/jamacardio.2024.4934. JAMA Cardiol. 2025. PMID: 39774481 Free PMC article. No abstract available.
Abstract
Importance: The differences between the use of fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) in the long term are unknown.
Objective: To compare long-term outcomes of iFR- and FFR-based strategies to guide revascularization.
Design, setting, and participants: The DEFINE-FLAIR multicenter study randomized patients with coronary artery disease to use either iFR or FFR as a pressure index to guide revascularization. Patients from 5 continents with coronary artery disease and angiographically intermediate severity stenoses who underwent hemodynamic interrogation with pressure wires were included. These data were analyzed from March, 13, 2014, through April, 27, 2021.
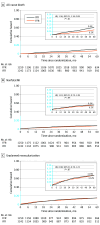
Main outcome measures: Five-year major adverse cardiac events (MACE) (a composite of all-cause death, nonfatal myocardial infarction, and unplanned revascularization), as well as the individual components of the combined end point.
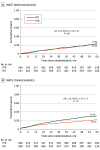
Results: At 5 years of follow-up, no significant differences were found between the iFR (mean age [SD], 65.5 [10.8] years; 962 male [77.5%]) and FFR (mean age [SD], 65.2 [10.6] years; 929 male [74.3%]) groups in terms of MACE (21.1% vs 18.4%, respectively; hazard ratio [HR], 1.18; 95% CI, 0.99-1.42; P = .06). While all-cause death was higher among patients randomized to iFR, it was not driven by myocardial infarction (6.3% vs 6.2% in the FFR study arm; HR, 1.01; 95% CI, 0.74-1.38; P = .94) or unplanned revascularization (11.9% vs 12.2% in the FFR group; HR, 0.98; 95% CI, 0.78-1.23; P = .87). Furthermore, patients in whom revascularization was deferred on the basis of iFR or FFR had similar MACE in both study arms (17.9% in the iFR group vs 17.5% in the FFR group; HR, 1.03; 95% CI, 0.79-1.35; P = .80) with similar rates of the components of MACE, including all-cause death. On the contrary, in patients who underwent revascularization after physiologic interrogation, the incidence of MACE was higher in the iFR group (24.6%) compared with the FFR group (19.2%) (HR, 1.36; 95% CI, 1.07-1.72; P = .01).
Conclusions and relevance: At 5-year follow up, an iFR based-strategy was not statistically different than an FFR strategy to guide revascularization in terms of MACE, nonfatal myocardial infarction, and unplanned revascularization.
Trial registration: ClinicalTrials.gov Identifier: NCT02053038.
Conflict of interest statement
Figures

Comment in
-
Parsing Signal vs Noise-Secondary Analyses of RCTs.JAMA Cardiol. 2025 Jan 1;10(1):31. doi: 10.1001/jamacardio.2024.3329. JAMA Cardiol. 2025. PMID: 39412815 No abstract available.
References
-
- Escaned J, Berry C, De Bruyne B, et al. Applied coronary physiology for planning and guidance of percutaneous coronary interventions. a clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology. EuroIntervention. 2023. 21;19(6):464-481. doi: 10.4244/EIJ-D-23-00194 - DOI - PMC - PubMed
-
- Pijls NHJ, van Son JAM, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354-1367. doi: 10.1161/01.CIR.87.4.1354 - DOI - PubMed
-
- Sen S, Escaned J, Malik IS, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59(15):1392-1402. doi: 10.1016/j.jacc.2011.11.003 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

