Organic-Inorganic Hybrid Materials from Vegetable Oils
- PMID: 39412784
- PMCID: PMC11628362
- DOI: 10.1002/marc.202400408
Organic-Inorganic Hybrid Materials from Vegetable Oils
Abstract
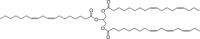
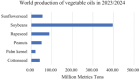
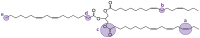
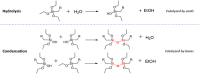
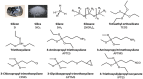
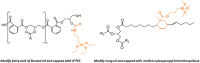
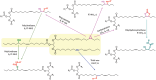
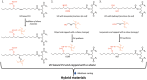
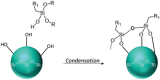
The production of materials based on fossil resources is yielding more sustainable and ecologically beneficial methods. Vegetable oils (VO) are one example of base materials whose derivatives rival the properties of their petro-based counterparts. Gaps exist however and one way to fill them is by employing sol-gel processes to synthesize organic-inorganic hybrid materials, often derived from silane/siloxane compounds. Creating Si─O─Si inorganic networks in the organic VO matrix permits the attainment of necessary strength, among other property enhancements. Consequently, many efforts have been directed to optimally achieve organic-inorganic hybrid materials with VOs. However, compatibilization is challenging, and desirable conditions for matching the inorganic filler in the organic matrix remain a key stumbling block toward wider application. Therefore, this review aims to detail recent progress on these new hybrids, focusing on the main strategies to polymerize and functionalize the raw VO, followed by routes highlighting the addition of the inorganic fillers to obtain desirable composites.
Keywords: hybrids; inorganic network; sol–gel process.
© 2024 The Author(s). Macromolecular Rapid Communications published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



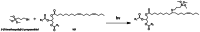


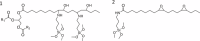
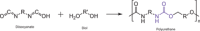

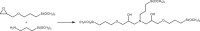
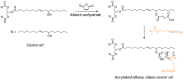
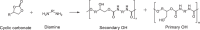
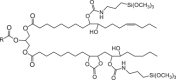
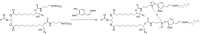
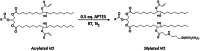


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

