Upper- vs Lower-Extremity Secondary Access During Transcatheter Aortic Valve Implantation: A Randomized Clinical Trial
- PMID: 39412806
- PMCID: PMC11581581
- DOI: 10.1001/jamanetworkopen.2024.38578
Upper- vs Lower-Extremity Secondary Access During Transcatheter Aortic Valve Implantation: A Randomized Clinical Trial
Abstract
Importance: An upper-extremity approach for secondary access during transfemoral transcatheter aortic valve implantation (TAVI) may reduce clinically relevant secondary access site-related bleeding.
Objective: To investigate the safety and efficacy of an upper-extremity approach compared with a lower-extremity approach in patients undergoing TAVI.
Design, setting, and participants: The TAVI XS trial was a randomized clinical trial performed between November 28, 2022, and November 15, 2023, with a 30-day follow-up, in 4 TAVI centers in the Netherlands. Eligibility was determined first, and only those patients with severe aortic stenosis and no contraindication for upper- or lower-extremity secondary access were informed about the study and asked to participate.
Intervention: Participants were randomized 1:1 between the upper-extremity approach (radial artery diagnostic access and upper-arm vein for temporary pacing lead placement) and lower-extremity approach (femoral artery diagnostic access and femoral vein for temporary pacing lead placement) for secondary access during TAVI.
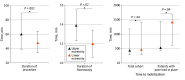
Main outcomes and measures: Primary end point was clinically relevant bleeding (Bleeding Academic Research Consortium type 2, 3, or 5) of the randomized secondary access. Secondary end points included any clinically relevant bleeding, time to mobilization, duration of hospitalization, secondary access failure, and procedural time.
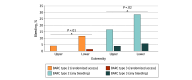
Results: Of a total of 324 eligible patients, 238 patients undergoing transfemoral TAVI (mean [SD] age, 79.4 [6.5] years; 150 male [63.0%]; median European System for Cardiac Operative Risk Evaluation II score, 2.2% [IQR, 1.5%-3.5%]) were included. The primary end point occurred in 5 of 119 patients (4.2%) in the upper-extremity group and 16 of 119 (13.4%) in the lower-extremity group (odds ratio [OR], 0.28 [95% CI, 0.10-0.80]; P = .01). Incidence of any clinically relevant bleeding was decreased in the upper-extremity group (25 of 119 [21.0%] vs 41 of 119 [34.5%] patients; OR, 0.51 [95% CI, 0.28-0.91]; P = .02). There was no difference in time to mobilization or duration of hospitalization. Secondary access failure (14 of 119 [11.8%] vs 1 of 119 [0.8%] patients; OR, 15.73 [95% CI, 2.03-121.69]; P = .001) and procedural time (60.0 [IQR, 39.0-88.0; 95% CI, 53.0-70.0] vs 48.0 [IQR, 34.0-64.0; 95% CI, 40.0-55.0] minutes; P = .002) were higher in the upper-extremity cohort.
Conclusion and relevance: In this randomized clinical trial of patients undergoing transfemoral TAVI, the upper-extremity approach for secondary access was associated with less clinically relevant access site-related bleeding compared with the conventional lower-extremity approach and should be considered to reduce periprocedural bleeding complications.
Trial registration: ClinicalTrials.gov Identifier: NCT05672823.
Conflict of interest statement
Figures

Comment in
-
Upper-Extremity Approach for Secondary Access in TAVI-Ready for Standard Clinical Practice?JAMA Netw Open. 2024 Oct 1;7(10):e2438529. doi: 10.1001/jamanetworkopen.2024.38529. JAMA Netw Open. 2024. PMID: 39412812 No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

