Lead Acetate Exposure and Cerebral Amyloid Accumulation: Mechanistic Evaluations in APP/PS1 Mice
- PMID: 39412896
- PMCID: PMC11482597
- DOI: 10.1289/EHP14384
Lead Acetate Exposure and Cerebral Amyloid Accumulation: Mechanistic Evaluations in APP/PS1 Mice
Abstract
Background: The role of environmental factors in Alzheimer's disease (AD) pathogenesis remains elusive. Mounting evidence suggests that acute and past exposure to the environmental toxicant lead (Pb) is associated with longitudinal decline in cognitive function, brain atrophy, and greater brain -amyloid () deposition. However, the nature of Pb-induced amyloid deposition and how it contributes to AD development remain unclear.
Objectives: This study investigates the role of Pb in the pathogenesis of cerebral amyloid angiopathy (CAA) and whether plasminogen activator inhibitor-1 (PAI-1) contributes to this process in the APP/PS1 mouse model.
Methods: Female APP/PS1 mice at 8 wk of age were administered either Pb-acetate (PbAc) (i.e., ) or an equivalent molar concentration of sodium acetate (NaAc) via oral gavage once daily for 8 wk. Amyloid deposition and vascular amyloid were determined by immunostaining. In addition, perivascular drainage, vascular binding assay, and microglial endocytosis were examined to determine underlying mechanisms. Furthermore, magnetic resonance imaging demyelination imaging was performed in vivo measure the level of demyelination. Finally, Y-maze and Morris water maze tests were assessed to evaluate the cognitive function of mice.
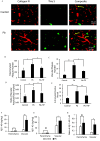
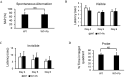
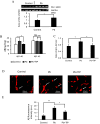
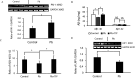
Results: APP/PS1 mice (an AD mice model) exposed to PbAc demonstrated more vascular amyloid deposition less neocortical myelination, and lower cognitive function, as well as greater vascular binding to , higher ratios, strikingly lower levels in the perivascular drainage, and microglial endocytosis. Importantly, exposure to a specific PAI-1 inhibitor, tiplaxtinin, which previously was reported to lower CAA pathology in mice, resulted in less CAA-related outcomes following PbAc exposure.
Discussion: Our findings suggest that PbAc induced CAA/AD pathogenesis via the PAI-1 signaling in the APP/PS1 mouse model, and the inhibition of PAI-1 could be a potential therapeutic target for PbAc-mediated CAA/AD disorders. https://doi.org/10.1289/EHP14384.
Figures



References
-
- Haraguchi T, Ishizu H, Takehisa Y, Kawai K, Yokota O, Terada S, et al. . 2001. Lead content of brain tissue in diffuse neurofibrillary tangles with calcification (DNTC): the possibility of lead neurotoxicity. Neuroreport 12(18):3887–3890, PMID: 11742204, 10.1097/00001756-200112210-00006. - DOI - PubMed
-
- Graves AB, van Duijn CM, Chandra V, Fratiglioni L, Heyman A, Jorm AF, et al. . 1991. Occupational exposures to solvents and lead as risk factors for Alzheimer’s disease: a collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20 Suppl 2:S58–S61, PMID: 1833355, 10.1093/ije/20.supplement_2.s58. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

