Programmed BRD9 Degradation and Hedgehog Signaling Activation via Silk-Based Core-Shell Microneedles Promote Diabetic Wound Healing
- PMID: 39413023
- PMCID: PMC11615742
- DOI: 10.1002/advs.202404130
Programmed BRD9 Degradation and Hedgehog Signaling Activation via Silk-Based Core-Shell Microneedles Promote Diabetic Wound Healing
Abstract
Wound healing impairment in diabetes mellitus is associated with an excessive inflammatory response and defective regeneration capability with suppressed Hedgehog (Hh) signaling. The bromodomain protein BRD9, a subunit of the non-canonical BAF chromatin-remodeling complex, is critical for macrophage inflammatory response. However, whether the epigenetic drug BRD9 degrader can attenuate the sustained inflammatory state of wounds in diabetes remains unclear. Without a bona fide immune microenvironment, Hh signaling activation fails to effectively rescue the suppressed proliferative ability of dermal fibroblasts and the vascularization of endothelial cells. Therefore, a silk-based core-shell microneedle (MN) patch is proposed to dynamically modulate the wound immune microenvironment and the regeneration process. Specifically, the BRD9 degrader released from the shell of the MNs mitigated the excessive inflammatory response in the early phase. Subsequently, the positively charged Hh signaling agonist is released from the negatively charged core of the silk fibroin nanofibers and promotes the phase transition from inflammation to regeneration, including re-epithelialization, collagen deposition, and angiogenesis. These findings suggest that the programmed silk-based core-shell MN patch is an ideal therapeutic strategy for effective skin regeneration in diabetic wounds.
Keywords: Hedgehog signaling; diabetes; microneedle patch; silk fibroin nanofibers; wound healing.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
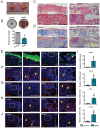
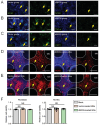
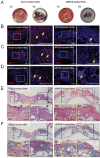
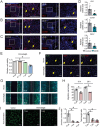



References
MeSH terms
Substances
Grants and funding
- 81921002/National Natural Science Foundation of China
- 82201004/National Natural Science Foundation of China
- 82130027/National Natural Science Foundation of China
- 81991505/National Natural Science Foundation of China
- 31900971/National Natural Science Foundation of China
- 82100966/National Natural Science Foundation of China
- YESS20230102/Young Elite Scientists Sponsorship Program by CAST
- 2023YFC2413600/National Key Research and Development Program of China
- 2022SKLS-KFKT008/Shanghai Key Laboratory of Stomatology, Shanghai Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine
- SHSMU-ZLCX20212400/Innovative Research Team of High-level Local University in Shanghai
LinkOut - more resources
Full Text Sources
