KCTD1 regulation of Adenylyl cyclase type 5 adjusts striatal cAMP signaling
- PMID: 39413138
- PMCID: PMC11513970
- DOI: 10.1073/pnas.2406686121
KCTD1 regulation of Adenylyl cyclase type 5 adjusts striatal cAMP signaling
Abstract
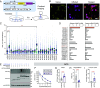
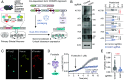
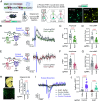
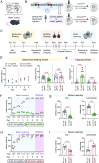
Dopamine transfers information to striatal neurons, and disrupted neurotransmission leads to motor deficits observed in movement disorders. Striatal dopamine converges downstream to Adenylyl Cyclase Type 5 (AC5)-mediated synthesis of cAMP, indicating the essential role of signal transduction in motor physiology. However, the relationship between dopamine decoding and AC5 regulation is unknown. Here, we utilized an unbiased global protein stability screen to identify Potassium Channel Tetramerization Domain 1 (KCTD1) as a key regulator of AC5 level that is mechanistically tied to N-linked glycosylation. We then implemented a CRISPR/SaCas9 approach to eliminate KCTD1 in striatal neurons expressing a Förster resonance energy transfer (FRET)-based cAMP biosensor. 2-photon imaging of striatal neurons in intact circuits uncovered that dopaminergic signaling was substantially compromised in the absence of KCTD1. Finally, knockdown of KCTD1 in genetically defined dorsal striatal neurons significantly altered motor behavior in mice. These results reveal that KCTD1 acts as an essential modifier of dopaminergic signaling by stabilizing striatal AC5.
Keywords: KCTD1; cAMP; dopamine; motor learning; striatum.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Graybiel A. M., Aosaki T., Flaherty A. W., Kimura M., The basal ganglia and adaptive motor control. Science 265, 1826–1831 (1994). - PubMed
-
- Picconi B., et al. , Pathological synaptic plasticity in the striatum: Implications for Parkinson’s disease. Neurotoxicology 26, 779–783 (2005). - PubMed
-
- Brotchie J. M., Adjuncts to dopamine replacement: A pragmatic approach to reducing the problem of dyskinesia in Parkinson’s disease. Mov. Disord. 13, 871–876 (1998). - PubMed
-
- Jankovic J., Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert. Opin. Pharmacother. 17, 2461–2470 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

