Discrimination and Precision of Continuous Glucose Monitoring in Staging Children With Presymptomatic Type 1 Diabetes
- PMID: 39413240
- PMCID: PMC12086423
- DOI: 10.1210/clinem/dgae691
Discrimination and Precision of Continuous Glucose Monitoring in Staging Children With Presymptomatic Type 1 Diabetes
Abstract
Context: Staging and monitoring of presymptomatic type 1 diabetes includes the assessment for dysglycemia.
Objective: To assess the ability of continuous glucose monitoring (CGM) to differentiate between islet autoantibody-negative controls and early-stage type 1 diabetes and explore whether CGM classifiers predict progression to clinical diabetes.
Research design and methods: Children and adolescents participating in public health screening for islet autoantibodies in Bavaria, Germany, were invited to undergo CGM with Dexcom G6. In total, 118 participated and valid data was obtained from 97 [57 female; median age 10 (range 3-17) years], including 46 with stage 1, 18 with stage 2, and 33 with no islet autoantibodies.
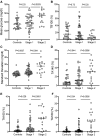
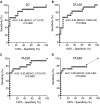
Results: Mean glucose during CGM in islet autoantibody-negative controls was high (median, 115.3 mg/dL) and varied substantially (interquartile range, 106.8-124.4). Eleven (33%) of the controls had more than 10% of glucose values above 140 mg/dL (TA140). Using thresholds corresponding to 100% specificity in controls, differences between controls and stage 1 and stage 2 were obtained for glucose SD, TA140, TA160, and TA180. Elevations in any 2 of these parameters identified 12 (67%) with stage 2 and 9 (82%) of 11 participants who developed clinical diabetes within 1 year. However, there was marked variation within groups for all parameters and poor consistency observed in a second CGM performed in 18 participants.
Conclusion: This study demonstrated the potential of integrating CGM into staging and monitoring of early-stage type 1 diabetes. However, substantial improvement in the precision of CGM is required for its application in routine monitoring practices.
Keywords: continuous glucose monitoring; disease progression; early-stage diagnosis; glucose variability; pediatrics; type 1 diabetes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

