The μ-opioid receptor differentiates two distinct human nociceptive populations relevant to clinical pain
- PMID: 39413733
- PMCID: PMC11513826
- DOI: 10.1016/j.xcrm.2024.101788
The μ-opioid receptor differentiates two distinct human nociceptive populations relevant to clinical pain
Abstract
The shortfall in new analgesic agents is a major impediment to reducing reliance on opioid medications for control of severe pain. In both animals and man, attenuating nociceptive transmission from primary afferent neurons with a μ-opioid receptor agonist yields highly effective analgesia. Consequently, deeper molecular characterization of human nociceptive afferents expressing OPRM1, the μ-opioid receptor gene, is a key component for advancing analgesic drug discovery and understanding clinical pain control. A co-expression matrix for the μ-opioid receptor and a variety of nociceptive channels as well as δ- and κ-opioid receptors is established by multiplex in situ hybridization. Our results indicate an OPRM1-positive population with strong molecular resemblance to rodent peptidergic C-nociceptors associated with tissue damage pain and an OPRM1-negative population sharing molecular characteristics of murine non-peptidergic C-nociceptors. The empirical identification of two distinct human nociceptive populations that differ profoundly in their presumed responsiveness to opioids provides an actionable translational framework for human pain control.
Keywords: MRGPRD; TRPA1; TRPV1; human nociception; neuropathic pain; non-opioid analgesics; sodium channels; somatosensory afferent neurons; translational research; μ-opioid receptor.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests A.G. is an employee and shareholder of AnaBios Corp.
Figures
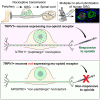

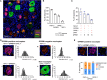


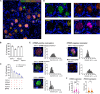


References
-
- Stein C., Zöllner C. Opioids and sensory nerves. Sensory Nerves. 2009:495–518. - PubMed
-
- Inturrisi C.E. Clinical Pharmacology of Opioids for Pain. Clin. J. Pain. 2002;18:S3–S13. - PubMed
-
- Benyamin R., Trescot A.M., Datta S., Buenaventura R., Adlaka R., Sehgal N., Glaser S.E., Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

